The Most Reactive Group In The Periodic Table
listenit
Apr 07, 2025 · 6 min read
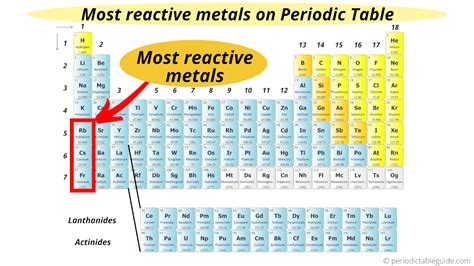
Table of Contents
The Most Reactive Group in the Periodic Table: The Alkali Metals
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic structure and properties. Among the diverse array of elements, certain groups exhibit strikingly similar characteristics. One such group, renowned for its exceptional reactivity, is the alkali metals – the stars of this exploration into the most reactive elements. Located in Group 1 (IA) of the periodic table, these elements—lithium (Li), sodium (Na), potassium (K), rubidium (Rb), cesium (Cs), and francium (Fr)—demonstrate a remarkable propensity for chemical reactions, shaping their unique properties and applications.
Understanding Reactivity: A Dive into Atomic Structure
The exceptional reactivity of alkali metals stems directly from their electronic configuration. Each alkali metal possesses a single electron in its outermost shell, also known as the valence shell. This solitary electron is relatively loosely held, making it incredibly easy to remove. This ease of electron removal is the key to understanding their high reactivity. The tendency to lose this electron to achieve a stable, filled outer electron shell (like the noble gases) is what drives their chemical behavior.
Ionization Energy: The Energy Barrier to Reactivity
Ionization energy is the energy required to remove an electron from a neutral atom. For alkali metals, the ionization energy is remarkably low. This low energy barrier means that very little energy is needed to remove the single valence electron, leading to the formation of a positively charged ion (cation) with a +1 charge. This ease of ionization is directly proportional to their reactivity. The lower the ionization energy, the higher the reactivity.
Electronegativity: A Measure of Electron Attraction
Electronegativity reflects an atom's ability to attract electrons in a chemical bond. Alkali metals have very low electronegativity values. This means they have a weak attraction for electrons, making them readily inclined to lose their valence electron rather than gain one to achieve a stable octet. This further contributes to their high reactivity.
The Chemistry of Reactivity: Reactions with Various Substances
The high reactivity of alkali metals translates to vigorous reactions with a wide range of substances. Let's examine some key reactions:
1. Reaction with Water: A Dramatic Display
The reaction of alkali metals with water is perhaps the most visually striking demonstration of their reactivity. When an alkali metal, like sodium, is added to water, it reacts violently. The metal rapidly oxidizes, losing its valence electron to water molecules. This electron transfer produces hydrogen gas and hydroxide ions, leading to the formation of an alkaline solution (high pH). The reaction is highly exothermic, releasing a significant amount of heat, often causing the hydrogen gas to ignite spontaneously, creating a spectacular burst of flame.
The vigor of this reaction increases as you move down the group. Lithium reacts relatively slowly, while sodium reacts more vigorously, potassium even more so, and rubidium and cesium react with explosive violence. This trend reflects the decreasing ionization energy as you descend the group, leading to an increasingly facile electron transfer.
The Equation: A Closer Look
The general equation for the reaction of an alkali metal (M) with water is:
2M(s) + 2H₂O(l) → 2M⁺(aq) + 2OH⁻(aq) + H₂(g)
2. Reaction with Oxygen: Formation of Oxides and Peroxides
Alkali metals react readily with oxygen in the air, forming various oxides. Lithium forms lithium oxide (Li₂O), while sodium forms primarily sodium oxide (Na₂O) along with some sodium peroxide (Na₂O₂). Potassium, rubidium, and cesium predominantly form superoxides (e.g., KO₂, RbO₂, CsO₂), showcasing a slightly different oxidation pattern. These reactions highlight the strong reducing power of alkali metals—their tendency to lose electrons and reduce other elements.
3. Reaction with Halogens: Salt Formation
Alkali metals react vigorously with halogens (Group 17 elements like fluorine, chlorine, bromine, and iodine) to form ionic salts. These reactions are highly exothermic and produce a bright flame, the color of which depends on the metal involved. For instance, the reaction of sodium with chlorine produces sodium chloride (NaCl), commonly known as table salt. The formation of these ionic compounds is driven by the electrostatic attraction between the positively charged alkali metal cation and the negatively charged halide anion.
The Significance of Ionic Bonding
The formation of ionic compounds is a crucial aspect of alkali metal reactivity. The large difference in electronegativity between the alkali metal (low) and the halogen (high) facilitates the transfer of an electron from the alkali metal to the halogen, forming stable ionic bonds.
4. Reaction with Acids: Further Demonstrations of Reactivity
Alkali metals react violently with acids, producing hydrogen gas and a salt. The reaction is even more vigorous than with water due to the higher concentration of H⁺ ions in the acidic solution. This reaction again underscores their strong reducing power and ease of oxidation.
Applications: Harnessing the Power of Alkali Metals
Despite their high reactivity, alkali metals find various applications, exploiting their unique properties:
-
Lithium-ion batteries: Lithium's high electrochemical potential makes it ideal for use in rechargeable batteries, powering many portable electronic devices and electric vehicles.
-
Sodium Vapor Lamps: Sodium vapor lamps, commonly used in street lighting, utilize the characteristic yellow-orange light emitted by excited sodium atoms.
-
Potassium in Fertilizers: Potassium is an essential nutrient for plant growth and is a vital component in many fertilizers.
-
Cesium in Atomic Clocks: Cesium's precise atomic transitions are utilized in highly accurate atomic clocks.
Safety Precautions: Handling Reactive Metals
The high reactivity of alkali metals demands careful handling and storage. They must be kept under anhydrous conditions (free from water) to prevent dangerous reactions. They are typically stored under mineral oil or inert atmospheres to prevent contact with air and moisture. Appropriate personal protective equipment (PPE), including gloves, goggles, and lab coats, is crucial when handling these materials.
Conclusion: The Reign of Reactivity
The alkali metals, with their single valence electron and low ionization energy, stand out as the most reactive group in the periodic table. Their vigorous reactions with various substances, including water, oxygen, halogens, and acids, highlight their exceptional reducing power and the strong driving force towards achieving a stable octet configuration. Understanding their reactivity is fundamental to comprehending their diverse applications, ranging from energy storage to agriculture and precision timekeeping. However, their potent reactivity also demands careful handling and stringent safety precautions to prevent accidents. The alkali metals, in their unique reactivity, offer a fascinating window into the fundamental principles of chemical bonding and reactivity, showcasing the intricate interplay of atomic structure and chemical behavior. Further research into these elements continues to unveil new applications and deepen our understanding of their remarkable properties.
Latest Posts
Latest Posts
-
Balance The Equation Ch4 O2 Co2 H2o
Apr 07, 2025
-
Is Mg A Cation Or Anion
Apr 07, 2025
-
What Is One Half Of A Quart
Apr 07, 2025
-
The Atomic Number Is The Same As The Number Of
Apr 07, 2025
-
If A Substance Is Ionic Then It Likely Will
Apr 07, 2025
Related Post
Thank you for visiting our website which covers about The Most Reactive Group In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.