Draw The Product Of The Hydration Of 2 Butene
listenit
Mar 17, 2025 · 5 min read
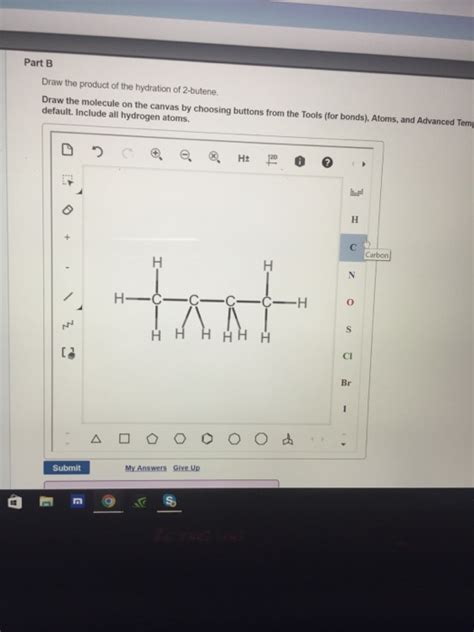
Table of Contents
Drawing the Product of the Hydration of 2-Butene: A Comprehensive Guide
The hydration of 2-butene is a classic example of an electrophilic addition reaction, a cornerstone concept in organic chemistry. Understanding this reaction not only illuminates fundamental principles but also provides a strong foundation for tackling more complex organic synthesis problems. This article will delve deep into the hydration of 2-butene, exploring the mechanism, predicting the product, and discussing the factors influencing the reaction's outcome. We'll also touch upon stereochemistry and its role in determining the final product's structure.
Understanding the Reaction: Hydration of Alkenes
Hydration, in the context of organic chemistry, refers to the addition of water (H₂O) across a double bond (alkene). This reaction is typically catalyzed by an acid, such as sulfuric acid (H₂SO₄) or phosphoric acid (H₃PO₄). The acid protonates the alkene, making it more susceptible to nucleophilic attack by water. The overall reaction is an addition reaction, meaning that the reactants combine to form a single product without the loss of any atoms.
2-Butene: The Starting Material
2-Butene is an alkene with the molecular formula C₄H₈. It exists as two geometric isomers: cis-2-butene and trans-2-butene. These isomers differ in the spatial arrangement of their substituents around the double bond. Understanding this isomerism is crucial because it influences the stereochemistry of the hydration product.
The Mechanism: A Step-by-Step Approach
The acid-catalyzed hydration of 2-butene proceeds through a three-step mechanism:
Step 1: Protonation of the Alkene
The acid catalyst (e.g., H₂SO₄) donates a proton (H⁺) to the double bond of 2-butene. This step forms a carbocation intermediate. Because 2-butene is a symmetrical alkene, protonation can occur at either carbon atom of the double bond, leading to the same carbocation intermediate (a secondary carbocation).
Step 2: Nucleophilic Attack by Water
A water molecule acts as a nucleophile, attacking the positively charged carbon atom of the carbocation. This step forms a protonated alcohol intermediate.
Step 3: Deprotonation
A base (often a water molecule or the conjugate base of the acid catalyst) removes a proton from the protonated alcohol, yielding the final product: 2-butanol.
Predicting the Product: 2-Butanol
The hydration of cis-2-butene or trans-2-butene both yield 2-butanol as the major product. However, the stereochemistry of the starting material influences the stereochemistry of the resulting 2-butanol.
Stereochemistry and Regiochemistry:
-
Regiochemistry: The addition of water to 2-butene follows Markovnikov's rule. This rule states that in the addition of a protic acid to an alkene, the hydrogen atom adds to the carbon atom that already has the greater number of hydrogen atoms. In the case of 2-butene, this results in the formation of 2-butanol, not 1-butanol.
-
Stereochemistry: The stereochemistry of the product depends on the stereochemistry of the starting alkene. The hydration of cis-2-butene predominantly yields racemic 2-butanol (a mixture of equal amounts of (R)-2-butanol and (S)-2-butanol). This is because the carbocation intermediate is planar, allowing for attack by water from either side with equal probability. Similarly, the hydration of trans-2-butene will also produce racemic 2-butanol, albeit potentially through a slightly different transition state.
Drawing the Product: A Step-by-Step Illustration
Let's illustrate the drawing of the product, 2-butanol, step-by-step:
-
Start with the carbon skeleton: Draw a four-carbon chain.
-
Add the hydroxyl group: Attach a hydroxyl group (-OH) to the second carbon atom. This reflects the Markovnikov addition.
-
Add the remaining hydrogen atoms: Complete the structure by adding hydrogen atoms to satisfy the tetravalency of each carbon atom.
The final structure should clearly show the -OH group attached to the second carbon atom of the butane chain. The structural formula is CH₃CH(OH)CH₂CH₃. Remember that 2-butanol exists as a pair of enantiomers, (R)-2-butanol and (S)-2-butanol, unless a chiral catalyst was used.
Factors Affecting the Reaction
Several factors can influence the hydration reaction:
-
Acid Catalyst: The choice of acid catalyst affects the reaction rate and potentially the selectivity. Stronger acids generally lead to faster reactions.
-
Temperature: Higher temperatures generally increase the reaction rate but may also lead to side reactions.
-
Solvent: The solvent can influence the reaction rate and selectivity. Polar protic solvents are often preferred.
-
Concentration of Reactants: Higher concentrations of both 2-butene and water generally lead to faster reactions.
Applications and Significance
The hydration of alkenes, including 2-butene, has significant applications in industrial chemistry:
-
Production of Alcohols: Alcohols are valuable chemicals used as solvents, fuels, and intermediates in the synthesis of other compounds. The hydration of alkenes provides a direct and efficient route to their production.
-
Fine Chemical Synthesis: The reaction is frequently employed in the synthesis of complex organic molecules in pharmaceutical and other fine chemical industries.
Conclusion
The hydration of 2-butene is a fundamental reaction in organic chemistry that provides a clear illustration of electrophilic addition and Markovnikov's rule. Understanding the mechanism and stereochemical aspects of this reaction allows us to predict the product – 2-butanol – and its properties. This knowledge forms a crucial basis for understanding more complex organic reactions and synthetic strategies. The reaction's industrial significance further underscores its importance in chemical manufacturing and the broader field of chemistry. Further explorations could include analyzing specific reaction conditions and their influence on reaction yield and selectivity. Furthermore, studying the use of chiral catalysts to obtain enantiomerically pure 2-butanol would also deepen understanding of this essential reaction.
Latest Posts
Latest Posts
-
What Is 1 2 3 As A Decimal
Mar 17, 2025
-
What Is 1 And 2 3 As A Decimal
Mar 17, 2025
-
What Is The Electron Configuration Of Argon
Mar 17, 2025
-
Is Chlorine A Solid Liquid Or Gas
Mar 17, 2025
-
What Is The Lcm For 7 And 9
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about Draw The Product Of The Hydration Of 2 Butene . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
