Does Nh3 Have Dipole Dipole Forces
listenit
Mar 18, 2025 · 6 min read
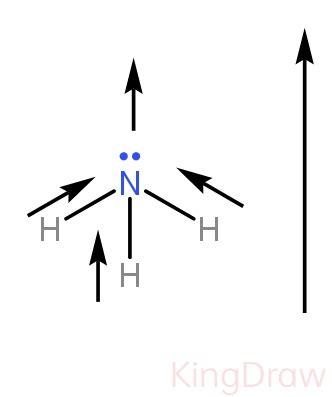
Table of Contents
Does NH₃ Have Dipole-Dipole Forces? A Deep Dive into Ammonia's Intermolecular Interactions
Ammonia (NH₃), a ubiquitous compound in nature and industry, presents a fascinating case study in intermolecular forces. Understanding its interactions is crucial to comprehending its properties, from its boiling point to its solubility. A key question often arises: Does NH₃ have dipole-dipole forces? The short answer is yes, but a comprehensive understanding requires a closer look at its molecular geometry and the nature of these forces.
Understanding Dipole-Dipole Forces
Before delving into ammonia's specifics, let's establish a foundational understanding of dipole-dipole forces. These forces are a type of intermolecular force that occurs between polar molecules. A polar molecule possesses a permanent dipole moment, meaning it has a slightly positive end and a slightly negative end due to an uneven distribution of electron density. This uneven distribution arises from differences in electronegativity between the atoms within the molecule.
Electronegativity is the ability of an atom to attract electrons within a chemical bond. When atoms with significantly different electronegativities bond, the electrons are pulled more towards the more electronegative atom, creating a partial negative charge (δ-) on that atom and a partial positive charge (δ+) on the less electronegative atom. This charge separation creates the dipole moment.
Dipole-dipole forces are the electrostatic attractions between the positive end of one polar molecule and the negative end of another. These forces are relatively strong compared to other intermolecular forces like London dispersion forces, but weaker than ionic or covalent bonds.
The Molecular Geometry of Ammonia (NH₃)
Ammonia's molecular structure is crucial in determining the presence and strength of dipole-dipole forces. The central nitrogen atom is bonded to three hydrogen atoms, and there is one lone pair of electrons on the nitrogen atom. This results in a trigonal pyramidal molecular geometry.
The nitrogen atom is significantly more electronegative than the hydrogen atoms. This electronegativity difference leads to a polar N-H bond, with the nitrogen atom carrying a partial negative charge (δ-) and the hydrogen atoms carrying partial positive charges (δ+). The presence of the lone pair on the nitrogen atom further contributes to the molecule's overall polarity.
This asymmetrical distribution of charge, stemming from the trigonal pyramidal geometry and the electronegativity difference, results in a significant net dipole moment for the ammonia molecule. This net dipole moment confirms the presence of polarity in the molecule, making it susceptible to dipole-dipole interactions.
Visualizing the Dipole Moment
Imagine the ammonia molecule as a pyramid with the nitrogen atom at the apex and the hydrogen atoms at the base. The nitrogen atom with its partial negative charge is at the top, and the hydrogen atoms with their partial positive charges form the base. This clearly demonstrates the molecule's polar nature. The vector sum of the individual bond dipoles results in a net dipole moment pointing from the base (H atoms) towards the apex (N atom).
The Role of Hydrogen Bonding in NH₃
While ammonia exhibits dipole-dipole forces, it's also crucial to acknowledge the significant role of hydrogen bonding. Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom is bonded to a highly electronegative atom (like nitrogen, oxygen, or fluorine) and is attracted to another electronegative atom in a nearby molecule.
In ammonia, the hydrogen atoms, carrying a partial positive charge (δ+), are attracted to the lone pairs of electrons on the nitrogen atoms of neighboring ammonia molecules. This strong attractive force is the hydrogen bond. Hydrogen bonding is responsible for many of ammonia's unique properties, such as its relatively high boiling point compared to other molecules of similar molar mass.
It's important to understand that hydrogen bonding is a special type of dipole-dipole interaction, not a separate category entirely. It arises from the strong electronegativity difference and the small size of the hydrogen atom, allowing for a closer interaction between molecules.
Distinguishing Dipole-Dipole and Hydrogen Bonding in NH₃
While hydrogen bonding is a type of dipole-dipole interaction, it's crucial to distinguish between them in the context of ammonia. The dipole-dipole interactions in ammonia refer to the general electrostatic attractions between the positive end of one molecule and the negative end of another. Hydrogen bonding is a stronger subclass of these interactions, specifically focusing on the interactions involving hydrogen atoms bonded to highly electronegative atoms. Ammonia exhibits both general dipole-dipole interactions and the stronger hydrogen bonding interactions.
Comparing NH₃ with Other Molecules
To further understand the significance of dipole-dipole forces and hydrogen bonding in ammonia, let's compare it to other molecules:
-
Methane (CH₄): Methane is a nonpolar molecule with a tetrahedral geometry. The electronegativity difference between carbon and hydrogen is negligible, resulting in no net dipole moment and no dipole-dipole forces. Its intermolecular forces are limited to weak London dispersion forces.
-
Water (H₂O): Water, like ammonia, is a polar molecule. It exhibits both dipole-dipole forces and strong hydrogen bonding. The bent geometry of water enhances its polarity, making its hydrogen bonding even more significant than in ammonia.
-
Hydrogen Chloride (HCl): HCl is a polar molecule with dipole-dipole interactions. However, it lacks the strong hydrogen bonding present in ammonia and water because chlorine, although electronegative, is not as electronegative as nitrogen or oxygen.
Consequences of Dipole-Dipole Forces and Hydrogen Bonding in NH₃
The presence of dipole-dipole forces and particularly hydrogen bonding significantly influences ammonia's physical and chemical properties:
-
Boiling Point: Ammonia has a relatively high boiling point compared to other molecules of similar molar mass. This is directly attributable to the strong hydrogen bonding between ammonia molecules, requiring more energy to overcome these intermolecular attractions during vaporization.
-
Solubility: Ammonia is highly soluble in water. This is due to the strong hydrogen bonding between ammonia molecules and water molecules. The partial positive charges on the hydrogen atoms of ammonia interact favorably with the partial negative charges on the oxygen atoms of water, and vice versa.
-
Reactivity: The polarity of ammonia, including its hydrogen bonding capability, influences its reactivity. It readily participates in various chemical reactions, often acting as a base due to the availability of its lone pair of electrons.
Conclusion: A Comprehensive Understanding
In conclusion, NH₃ definitively possesses dipole-dipole forces. The presence of a net dipole moment due to the trigonal pyramidal geometry and the electronegativity difference between nitrogen and hydrogen guarantees these interactions. However, the importance of recognizing the significant contribution of hydrogen bonding, a stronger type of dipole-dipole interaction, cannot be overstated. This special type of interaction is primarily responsible for ammonia's unique properties such as its comparatively high boiling point and excellent water solubility. A complete understanding of ammonia's intermolecular forces requires consideration of both dipole-dipole forces and the powerful influence of hydrogen bonding. This knowledge is crucial for appreciating the diverse applications and chemical behavior of this vital compound.
Latest Posts
Latest Posts
-
What Is The Longest Phase In Mitosis
Mar 18, 2025
-
How Many Quarts Are In 7 Pints
Mar 18, 2025
-
Is Speed A Vector Or A Scalar
Mar 18, 2025
-
What Is The Valence Electrons Of Nitrogen
Mar 18, 2025
-
18 Out Of 24 As A Percentage
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Does Nh3 Have Dipole Dipole Forces . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
