Does A Gas Have A Definite Shape
listenit
Mar 27, 2025 · 5 min read
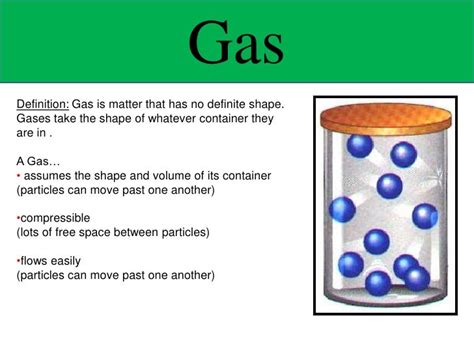
Table of Contents
Does a Gas Have a Definite Shape? Exploring the Properties of Gases
The question of whether a gas has a definite shape is fundamental to understanding the nature of matter. Unlike solids, which possess both definite shape and volume, and liquids, which have a definite volume but take the shape of their container, gases exhibit unique characteristics. This article will delve deep into the properties of gases, explaining why they lack a definite shape and exploring the microscopic behavior that dictates their macroscopic properties. We'll also discuss how pressure, temperature, and volume interact to define the state of a gas and examine real-world examples to solidify our understanding.
The Kinetic Molecular Theory: The Foundation of Gas Behavior
To understand why gases don't have a definite shape, we must first grasp the Kinetic Molecular Theory (KMT). This theory provides a microscopic model that explains the macroscopic properties of gases. The KMT postulates the following:
-
Gases are composed of tiny particles (atoms or molecules) that are in constant, random motion. This ceaseless movement is the driving force behind the lack of a definite shape. The particles are not static but are constantly colliding with each other and the walls of their container.
-
The volume of the particles themselves is negligible compared to the volume of the container. This means that the spaces between gas particles are vast compared to their size. This explains the compressibility of gases; you can squeeze them into smaller volumes because there's plenty of empty space.
-
The attractive and repulsive forces between gas particles are negligible. While some attractive forces exist between molecules (especially in polar gases), they are generally weak compared to the kinetic energy of the particles, especially at higher temperatures. This weak interaction allows the particles to move freely and independently.
-
Collisions between gas particles and between particles and the container walls are perfectly elastic. This means that no kinetic energy is lost during collisions; the total kinetic energy of the system remains constant.
-
The average kinetic energy of the particles is directly proportional to the absolute temperature. This explains why gases expand when heated; the increased kinetic energy leads to more vigorous particle motion, resulting in greater expansion.
These postulates directly explain why a gas does not have a definite shape. Because the particles are in constant, random motion and the forces between them are negligible, they readily adapt to the shape of any container they occupy. The particles fill the entire available space, adopting the container's form.
Pressure: A Consequence of Particle Motion
The constant motion of gas particles is responsible for another key property: pressure. Pressure is defined as the force exerted per unit area. In gases, this force arises from the billions of collisions of gas particles with the walls of their container. The more frequent and forceful these collisions, the higher the pressure.
This concept is directly related to the shape of a gas. Since the particles are moving randomly and colliding with all container surfaces, the pressure is distributed uniformly across the container. The gas expands to fill the entire available volume, resulting in a pressure that is uniform across all surfaces. This uniform pressure distribution means that a gas adopts the shape of its container.
Temperature and Volume: Their Role in Shaping Gas Behavior
Temperature and volume are also crucial factors influencing the behavior of gases. As mentioned earlier, temperature directly affects the kinetic energy of gas particles. A higher temperature means higher kinetic energy, leading to more frequent and forceful collisions with container walls, thus increasing pressure. If the container is flexible, this increased pressure will cause expansion, increasing the volume.
Conversely, decreasing the temperature reduces the kinetic energy, leading to slower particle movement and lower pressure. If the container is flexible, the gas will contract, decreasing its volume. This temperature dependence of volume further highlights the fact that gases conform to the shape of their containers. The volume adapts to the available space, dictated by the container's boundaries.
Real Gases vs. Ideal Gases
The KMT and the explanations above describe an ideal gas. An ideal gas perfectly adheres to the postulates of the KMT. However, real gases deviate from ideal behavior, particularly at high pressures and low temperatures.
At high pressures, the volume of the gas particles themselves becomes significant compared to the volume of the container, affecting the compressibility and, consequently, the pressure exerted. At low temperatures, the attractive forces between gas particles become more important, leading to deviations from ideal behavior.
These deviations, however, do not fundamentally change the fact that real gases still lack a definite shape. While the behavior of real gases is more complex, the underlying principle remains: the particles are in constant motion, and they will fill the available space, thereby taking the shape of the container.
Real-World Examples: Demonstrating the Lack of Definite Shape
Several everyday examples illustrate the indefinite shape of gases:
-
Air: The air in a room fills the entire space, taking on the room's shape. It doesn't have its own shape; it conforms to the boundaries of the room.
-
Helium balloons: Helium gas, being lighter than air, rises and fills the balloon, taking the spherical shape of the balloon's material. If the balloon were differently shaped, the helium would conform to that new shape.
-
Inflatable objects: Inflatable toys, mattresses, and tires demonstrate the same principle. The gas fills the object, taking on its shape. The gas molecules are not rigidly bound to each other; they are free to move around and distribute themselves throughout the available space.
-
Atmospheric pressure: The atmosphere itself is a vast expanse of gas. It encompasses the entire planet, conforming to the Earth's shape, but not having a definite shape of its own.
Conclusion: Shape Defined by the Container
In summary, a gas does not possess a definite shape. Its shape is completely dictated by the container it occupies. This is a direct consequence of the Kinetic Molecular Theory, where gas particles are in constant, random motion, have negligible intermolecular forces, and readily adapt to the available volume. While real gases deviate from ideal behavior under certain conditions, the fundamental principle – the lack of a definite shape – remains true. The particles will always strive to fill the entire container, adopting its shape and thus uniformly distributing the pressure exerted on all container surfaces. Understanding this fundamental property of gases is essential for comprehending numerous physical phenomena and technological applications involving gases.
Latest Posts
Latest Posts
-
0 6 As A Fraction In Simplest Form
Mar 30, 2025
-
What Is The Only Liquid Layer Of The Earth
Mar 30, 2025
-
What The Molecular Shape Geometry Of Chclo
Mar 30, 2025
-
What Is The Atomic Number Of An Atom Equivalent To
Mar 30, 2025
-
Diameter Of Solar System In Light Years
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about Does A Gas Have A Definite Shape . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
