Do Ionic Bonds Have High Melting Points
listenit
Mar 24, 2025 · 6 min read
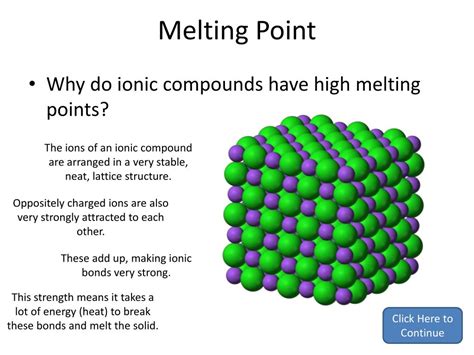
Table of Contents
Do Ionic Bonds Have High Melting Points? A Deep Dive into Ionic Compounds
Ionic bonds, characterized by the electrostatic attraction between oppositely charged ions, are responsible for many fascinating properties of the resulting compounds. One of the most striking characteristics often associated with ionic compounds is their high melting points. But why is this the case? Let's delve deep into the nature of ionic bonds and explore the reasons behind their impressive thermal stability.
Understanding Ionic Bonds: A Foundation for High Melting Points
Before we tackle the melting point phenomenon, it's crucial to grasp the fundamental principles of ionic bonding. Ionic bonds are formed when a metal atom, typically possessing low ionization energy and a tendency to lose electrons, interacts with a non-metal atom, which possesses high electron affinity and a tendency to gain electrons. This transfer of electrons creates positively charged cations (metal ions) and negatively charged anions (non-metal ions).
The electrostatic force of attraction between these oppositely charged ions is exceptionally strong, forming a stable ionic lattice structure. This lattice isn't a random arrangement; rather, it's a highly ordered, three-dimensional structure where each ion is surrounded by several ions of the opposite charge, maximizing electrostatic attraction and minimizing repulsion. This precise arrangement is key to understanding the high melting points.
The Role of Electrostatic Forces: The Glue Holding Ionic Compounds Together
The strength of the ionic bond is directly proportional to the charge of the ions and inversely proportional to the distance between them. This relationship is elegantly described by Coulomb's Law:
F = k * |q1 * q2| / r²
Where:
- F represents the electrostatic force
- k is Coulomb's constant
- q1 and q2 are the charges of the ions
- r is the distance between the ions
The larger the charges of the ions and the smaller the distance between them, the stronger the electrostatic attraction. This strong attraction is the primary reason why ionic compounds possess high melting points.
Melting Point: Overcoming the Strong Electrostatic Forces
The melting point of a substance is the temperature at which it transitions from a solid state to a liquid state. For ionic compounds, this transition requires overcoming the strong electrostatic forces holding the ions together in the lattice structure. A significant amount of energy, in the form of heat, is needed to disrupt this highly ordered arrangement and allow the ions to move more freely, characteristic of the liquid phase.
The Energy Barrier: Breaking the Lattice Structure
The energy required to break the ionic bonds and melt the compound is directly related to the strength of the electrostatic forces within the lattice. Stronger bonds necessitate more energy input to overcome the attractive forces, resulting in a higher melting point. Conversely, weaker bonds require less energy, leading to lower melting points.
Factors Influencing the Melting Point of Ionic Compounds
Several factors contribute to the variation in melting points observed across different ionic compounds:
-
Charge of the Ions: Compounds with ions carrying higher charges (e.g., Al³⁺ and O²⁻ in Al₂O₃) exhibit stronger electrostatic attractions compared to compounds with ions carrying lower charges (e.g., Na⁺ and Cl⁻ in NaCl). This leads to significantly higher melting points for compounds with highly charged ions.
-
Size of the Ions: Smaller ions are more closely packed in the lattice structure, resulting in shorter distances between ions and thus stronger electrostatic attractions. Larger ions, on the other hand, are further apart, weakening the attractive forces and lowering the melting point.
-
Lattice Structure: The specific arrangement of ions in the lattice also influences the melting point. Different lattice structures have varying degrees of efficiency in packing ions, influencing the overall strength of the electrostatic interactions. Compounds with more efficient packing arrangements generally have higher melting points.
-
Polarizability: While primarily related to covalent bonds, polarizability can subtly influence ionic compounds. If one of the ions is highly polarizable, it can lead to slight distortions in the electron cloud, affecting the overall strength of the electrostatic interactions and subsequently influencing the melting point.
Examples and Comparisons: Illustrating the Trend
Let's examine some specific examples to illustrate the relationship between ionic bonding and high melting points:
-
Sodium Chloride (NaCl): A classic example of an ionic compound, NaCl has a relatively high melting point of 801°C. This is attributed to the relatively strong electrostatic attraction between the Na⁺ and Cl⁻ ions.
-
Magnesium Oxide (MgO): With Mg²⁺ and O²⁻ ions carrying higher charges than Na⁺ and Cl⁻, MgO boasts a significantly higher melting point of 2852°C. The increased charge leads to stronger electrostatic interactions.
-
Aluminum Oxide (Al₂O₃): Possessing even higher charged ions (Al³⁺ and O²⁻), Al₂O₃ exhibits an exceptionally high melting point of over 2000°C. The intense electrostatic forces require a substantial amount of energy to break.
These examples clearly demonstrate the correlation between the charge of the ions and the melting point. The higher the charge, the stronger the electrostatic attraction, and consequently, the higher the melting point.
Exceptions and Deviations: When Ionic Compounds Have Lower Melting Points
While most ionic compounds exhibit high melting points, exceptions exist. Certain factors can lead to deviations from the general trend:
-
Large Ion Size: Compounds with exceptionally large ions may have lower melting points due to the increased distance between ions, weakening electrostatic attraction.
-
Covalent Character: Some ionic compounds may exhibit a degree of covalent character, which can weaken the overall ionic bonding and lower the melting point. This is particularly relevant in compounds where there's a significant difference in electronegativity between the metal and non-metal, leading to a less purely ionic bond.
-
Complex Ions: The presence of complex ions can sometimes disrupt the regular lattice structure and reduce the overall strength of electrostatic interactions, impacting the melting point.
-
Lattice Defects: Imperfections in the crystal lattice structure can also affect the melting point. These defects can disrupt the regular arrangement of ions, reducing the strength of the overall lattice and lowering the melting point.
Conclusion: The Significance of Ionic Bonding and High Melting Points
The high melting points of ionic compounds are a direct consequence of the strong electrostatic forces of attraction between oppositely charged ions within their highly ordered lattice structures. This property has significant implications in various applications:
-
Materials Science: Ionic compounds with high melting points are used in high-temperature applications, such as ceramics, refractories, and protective coatings.
-
Industrial Processes: Many industrial processes utilize the thermal stability of ionic compounds.
-
Geochemistry: Understanding the melting points of ionic compounds is crucial for interpreting geological processes and mineral formation.
The relationship between ionic bonding and melting point is a fundamental concept in chemistry, highlighting the importance of electrostatic forces in determining the physical properties of matter. By understanding the factors influencing the strength of ionic bonds, we can better predict and explain the thermal behavior of ionic compounds and harness their unique properties in diverse applications. Further research continues to refine our understanding of these interactions and their impact on the properties of materials.
Latest Posts
Latest Posts
-
Find The Differential Of The Function
Mar 26, 2025
-
In A Double Covalent Bond A Carbon Atom Shares
Mar 26, 2025
-
15 Of 60 Is What Number
Mar 26, 2025
-
Can Two Different Numbers Have The Same Absolute Value
Mar 26, 2025
-
How Many Neutrons Are In C14
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Do Ionic Bonds Have High Melting Points . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
