Condensed Structural Formula Vs Molecular Formula
listenit
Mar 22, 2025 · 5 min read
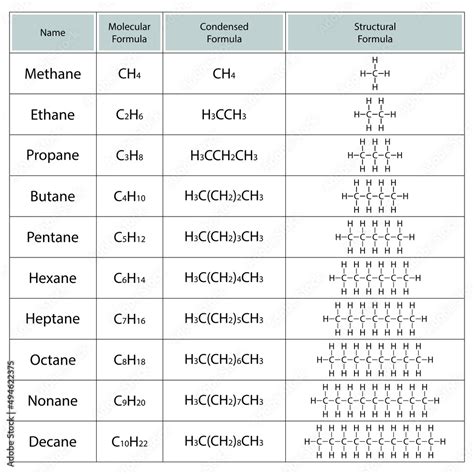
Table of Contents
- Condensed Structural Formula Vs Molecular Formula
- Table of Contents
- Condensed Structural Formula vs. Molecular Formula: A Comprehensive Guide
- What is a Molecular Formula?
- What is a Condensed Structural Formula?
- Comparing Condensed Structural Formulas and Molecular Formulas: A Detailed Comparison Table
- When to Use Which Formula?
- Beyond Molecular and Condensed Formulas: Other Representations
- Conclusion: Mastering Molecular Representations for Chemical Understanding
- Latest Posts
- Latest Posts
- Related Post
Condensed Structural Formula vs. Molecular Formula: A Comprehensive Guide
Understanding the structure of molecules is fundamental in chemistry. While both condensed structural formulas and molecular formulas convey information about the composition of a molecule, they differ significantly in the level of detail they provide. This article delves into the nuances of these two representations, highlighting their strengths, weaknesses, and practical applications. We'll explore how they relate to each other and why choosing the appropriate formula is crucial depending on the context.
What is a Molecular Formula?
A molecular formula provides the simplest representation of a molecule, showing only the types and numbers of atoms present. It uses chemical symbols to indicate the elements and subscripts to denote the number of atoms of each element. For example:
- Water: H₂O (two hydrogen atoms and one oxygen atom)
- Methane: CH₄ (one carbon atom and four hydrogen atoms)
- Glucose: C₆H₁₂O₆ (six carbon atoms, twelve hydrogen atoms, and six oxygen atoms)
Advantages of Molecular Formulas:
- Simplicity: They are concise and easy to write and understand.
- Quantitative Information: They clearly indicate the elemental composition of the molecule, allowing for stoichiometric calculations.
- Suitable for Basic Calculations: Molecular formulas are essential for calculating molar mass and determining empirical formulas.
Disadvantages of Molecular Formulas:
- Lack of Structural Information: They provide no information about how the atoms are connected within the molecule. Isomers, molecules with the same molecular formula but different arrangements of atoms, cannot be distinguished using only the molecular formula.
- Limited Use in Organic Chemistry: For complex organic molecules, molecular formulas become unwieldy and fail to provide sufficient detail for understanding their properties and reactions.
What is a Condensed Structural Formula?
A condensed structural formula provides a more detailed representation than a molecular formula. It shows the arrangement of atoms within a molecule, indicating which atoms are bonded to each other. While it doesn't explicitly show every bond, it presents a more informative structure than the molecular formula. It achieves this by grouping atoms together that are bonded to the same atom.
Let's consider some examples:
- Methane: CH₄ (Molecular Formula) becomes CH₄ (Condensed Structural Formula - in this case, they are the same, as methane is simple)
- Ethane: C₂H₆ (Molecular Formula) becomes CH₃CH₃ (Condensed Structural Formula). This shows that each carbon atom is bonded to three hydrogen atoms and another carbon atom.
- Propanol: C₃H₈O (Molecular Formula) can be represented as CH₃CH₂CH₂OH or CH₃CH(OH)CH₃. This illustrates the importance of condensed formulas in distinguishing isomers. The first represents 1-propanol, and the second represents 2-propanol. Both have the same molecular formula but different structural formulas and properties.
- Butane: C₄H₁₀ (Molecular Formula) has two isomers: n-butane (CH₃CH₂CH₂CH₃) and isobutane (CH₃CH(CH₃)CH₃).
Advantages of Condensed Structural Formulas:
- Improved Structural Information: They provide a clearer picture of the molecule's structure compared to molecular formulas. This is especially important in distinguishing isomers.
- More Informative than Molecular Formulas: They show the connectivity of atoms, offering a better understanding of the molecule's properties and reactivity.
- Essential for Organic Chemistry: Condensed structural formulas are indispensable in organic chemistry for depicting complex organic molecules.
Disadvantages of Condensed Structural Formulas:
- Less Concise than Molecular Formulas: They are more lengthy and complex to write compared to molecular formulas, particularly for large molecules.
- Can Still Be Ambiguous: For very complex molecules, they can still be ambiguous without the use of other representations, like skeletal formulas or 3D models.
- May Require Interpretation: While more informative, they might still require some interpretation to visualize the complete 3D structure.
Comparing Condensed Structural Formulas and Molecular Formulas: A Detailed Comparison Table
| Feature | Molecular Formula | Condensed Structural Formula |
|---|---|---|
| Information Provided | Type and number of atoms only | Type, number, and connectivity of atoms |
| Complexity | Simple, concise | More complex, less concise |
| Isomers | Cannot distinguish between isomers | Can distinguish between isomers (most cases) |
| Structure | No structural information | Shows some structural information |
| Use in Organic Chemistry | Limited use, mainly for stoichiometric calculations | Essential for understanding and representing organic molecules |
| Examples | H₂O, C₆H₁₂O₆, CH₄ | CH₃CH₃, CH₃CH₂OH, CH₃CH(CH₃)CH₃ |
| Clarity | Highly clear but limited information. | Relatively clear; complexity increases with molecule size |
When to Use Which Formula?
The choice between a molecular formula and a condensed structural formula depends heavily on the context and the level of detail required:
-
Molecular formulas are best suited for:
- Stoichiometric calculations: Balancing chemical equations, determining molar masses, and performing other quantitative analyses.
- Simple molecules: Where the structure is straightforward and doesn't require detailed representation.
- Situations where conciseness is prioritized: Like labeling chemicals in a list or summary.
-
Condensed structural formulas are preferable for:
- Organic chemistry: Representing complex organic molecules where connectivity is crucial.
- Isomer distinction: Clearly showing differences in the arrangements of atoms within isomeric molecules.
- Understanding chemical reactions: Showing how atoms are rearranged during chemical reactions.
- Interpreting spectra: NMR and IR spectroscopy often require understanding the molecule's structure for interpretation.
Beyond Molecular and Condensed Formulas: Other Representations
While molecular and condensed structural formulas are essential, they are not the only ways to represent molecular structures. Other methods include:
- Skeletal formulas (line-angle formulas): These represent carbon atoms as intersections of lines and hydrogen atoms are implied. They're highly concise and commonly used in organic chemistry.
- 3D models: Space-filling models, ball-and-stick models, and other 3D representations provide a visual depiction of the molecule's three-dimensional structure, including bond angles and spatial arrangement.
Conclusion: Mastering Molecular Representations for Chemical Understanding
Choosing the correct representation of a molecule – whether it's a molecular formula, a condensed structural formula, or another method – is critical for clear communication and accurate interpretation in chemistry. Understanding the strengths and weaknesses of each representation ensures effective communication and allows for a deeper understanding of the molecule's properties and reactivity. The transition from simpler molecular formulas to more detailed condensed structural formulas (and beyond) mirrors the progression in understanding the complexities of chemical structure and its relationships to chemical behavior. Mastering these representations is crucial for success in chemistry at all levels.
Latest Posts
Latest Posts
-
Find Equation Of Plane Through Point And Parallel To Plane
Mar 24, 2025
-
What Is The Charge Of Cl
Mar 24, 2025
-
What Is Prime Factorization Of 63
Mar 24, 2025
-
Least Common Multiple 14 And 21
Mar 24, 2025
-
What Is The Lcm Of 8 12
Mar 24, 2025
Related Post
Thank you for visiting our website which covers about Condensed Structural Formula Vs Molecular Formula . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
