Calculating The Ph Of A Weak Acid Solution
listenit
Apr 01, 2025 · 6 min read
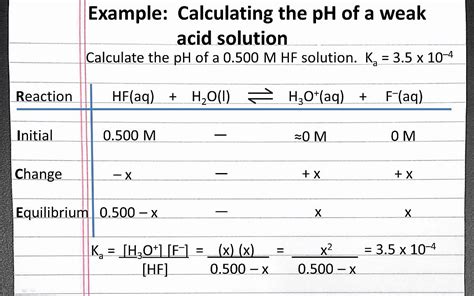
Table of Contents
Calculating the pH of a Weak Acid Solution: A Comprehensive Guide
Determining the pH of a solution is a fundamental concept in chemistry with wide-ranging applications, from environmental monitoring to medical diagnostics. While calculating the pH of a strong acid solution is relatively straightforward, calculating the pH of a weak acid solution requires a deeper understanding of equilibrium and dissociation constants. This comprehensive guide will delve into the methods and considerations involved in accurately calculating the pH of a weak acid solution.
Understanding Weak Acids and Their Dissociation
Unlike strong acids, which completely dissociate in water, weak acids only partially dissociate. This means that only a fraction of the acid molecules break down into their constituent ions (H⁺ and the conjugate base). The extent of dissociation is governed by the acid dissociation constant (Ka), which is an equilibrium constant that represents the ratio of the concentrations of the products (H⁺ and conjugate base) to the concentration of the undissociated acid at equilibrium.
A higher Ka value indicates a stronger weak acid (greater dissociation), while a lower Ka value indicates a weaker weak acid (less dissociation). The pKa, which is the negative logarithm of Ka (pKa = -log Ka), provides a more convenient scale for comparing acid strengths. A lower pKa value indicates a stronger acid.
The general equilibrium reaction for a weak acid (HA) is:
HA(aq) ⇌ H⁺(aq) + A⁻(aq)
The expression for the acid dissociation constant (Ka) is:
Ka = [H⁺][A⁻] / [HA]
Where:
- [H⁺] is the concentration of hydrogen ions (in mol/L)
- [A⁻] is the concentration of the conjugate base (in mol/L)
- [HA] is the concentration of the undissociated weak acid (in mol/L)
Methods for Calculating the pH of a Weak Acid Solution
Several methods exist for calculating the pH of a weak acid solution, depending on the complexity of the situation and the level of accuracy required.
1. The Approximation Method (for weak acids with low Ka)
This method simplifies the calculation by assuming that the extent of dissociation is small. This assumption allows us to neglect the concentration of H⁺ ions that come from the dissociation of the acid when calculating the concentration of the undissociated acid.
Steps:
-
Write the equilibrium expression: Begin by writing the equilibrium reaction and the corresponding Ka expression.
-
Create an ICE table: An ICE (Initial, Change, Equilibrium) table is crucial for organizing the concentrations.
| HA | H⁺ | A⁻ | |
|---|---|---|---|
| Initial | C | 0 | 0 |
| Change | -x | +x | +x |
| Equilibrium | C - x | x | x |
Where:
- C is the initial concentration of the weak acid.
- x is the change in concentration due to dissociation.
- Substitute into Ka expression: Substitute the equilibrium concentrations from the ICE table into the Ka expression:
Ka = x² / (C - x)
- Apply the approximation: Because x is typically small compared to C (for weak acids), we can simplify the equation to:
Ka ≈ x² / C
- Solve for x: Solve for x, which represents the [H⁺] concentration.
x = √(Ka * C)
- Calculate pH: Finally, calculate the pH using the formula:
pH = -log[H⁺] = -log(x)
Limitations: This approximation is only valid when the degree of dissociation is less than 5%. You should always verify this assumption after calculating x. If x/C > 0.05, the approximation is invalid, and a more accurate method (such as the quadratic formula) should be used.
2. The Quadratic Formula Method (for weak acids with higher Ka)
When the approximation method is invalid (x/C > 0.05), the quadratic formula must be used to solve the equilibrium expression accurately.
Steps:
-
Follow steps 1-3 from the approximation method. This involves writing the equilibrium expression and creating the ICE table.
-
Substitute into Ka expression: Substitute the equilibrium concentrations from the ICE table into the Ka expression:
Ka = x² / (C - x)
- Rearrange into quadratic form: Rearrange the equation into a standard quadratic equation (ax² + bx + c = 0):
x² + Kax - KaC = 0
- Solve using the quadratic formula: Use the quadratic formula to solve for x:
x = [-b ± √(b² - 4ac)] / 2a
Where:
- a = 1
- b = Ka
- c = -Ka*C
-
Choose the positive root: Only the positive root of the quadratic equation is physically meaningful since concentration cannot be negative.
-
Calculate pH: Calculate the pH using the formula:
pH = -log(x)
3. The Iterative Method (for complex scenarios)
For very complex scenarios involving polyprotic acids or mixtures of acids and bases, an iterative method may be necessary. This involves making an initial guess for the [H⁺] concentration, calculating the concentrations of other species based on that guess, and then refining the guess until the calculated [H⁺] converges to a consistent value. This method usually requires computational tools or software.
Factors Affecting the pH of Weak Acid Solutions
Several factors can influence the pH of a weak acid solution beyond the inherent strength of the acid itself:
-
Concentration of the weak acid: A higher concentration of weak acid leads to a lower pH (more acidic).
-
Temperature: The Ka of a weak acid changes with temperature. Generally, an increase in temperature increases the Ka, leading to a slightly lower pH.
-
Presence of common ions: The presence of a common ion (an ion already present in the solution, such as the conjugate base of the weak acid) reduces the dissociation of the weak acid, leading to a higher pH. This is described by the common ion effect.
-
Presence of other acids or bases: If other acids or bases are present in the solution, their contribution to the overall [H⁺] or [OH⁻] must be considered. This may require more complex calculations involving multiple equilibria.
Applications of Weak Acid pH Calculations
Accurate calculation of the pH of weak acid solutions is crucial in numerous applications:
-
Environmental science: Determining the acidity of rainwater, soil, or lakes is essential for understanding and mitigating environmental pollution.
-
Analytical chemistry: Many analytical techniques rely on controlling and precisely knowing the pH of the solutions used.
-
Medicine: The pH of bodily fluids is tightly regulated, and deviations can indicate various medical conditions.
-
Industrial processes: Many industrial processes, such as food processing and pharmaceutical production, require precise pH control.
Conclusion
Calculating the pH of a weak acid solution is a fundamental concept with broad implications. The appropriate method for calculation depends on the specific situation. While the approximation method offers simplicity for weak acids with low Ka, the quadratic formula provides more accuracy when the approximation breaks down. For more complex systems, iterative methods or computational tools might be necessary. Understanding these methods and the factors that influence pH is critical for accurate calculations and interpretation in various scientific and practical applications. Remember always to check the validity of your approximations and to consider the limitations of each method.
Latest Posts
Latest Posts
-
X 3 2x 2 X 2 0
Apr 02, 2025
-
1 3 Divided By 1 6 As A Fraction
Apr 02, 2025
-
How To Determine Zeros Of A Function
Apr 02, 2025
-
What Are Four Principles Of Natural Selection
Apr 02, 2025
-
What Is 9 To The Power Of 0
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Calculating The Ph Of A Weak Acid Solution . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
