Calculate The Mass In Grams Of 0.0420 Mol Of Copper.
listenit
May 12, 2025 · 5 min read
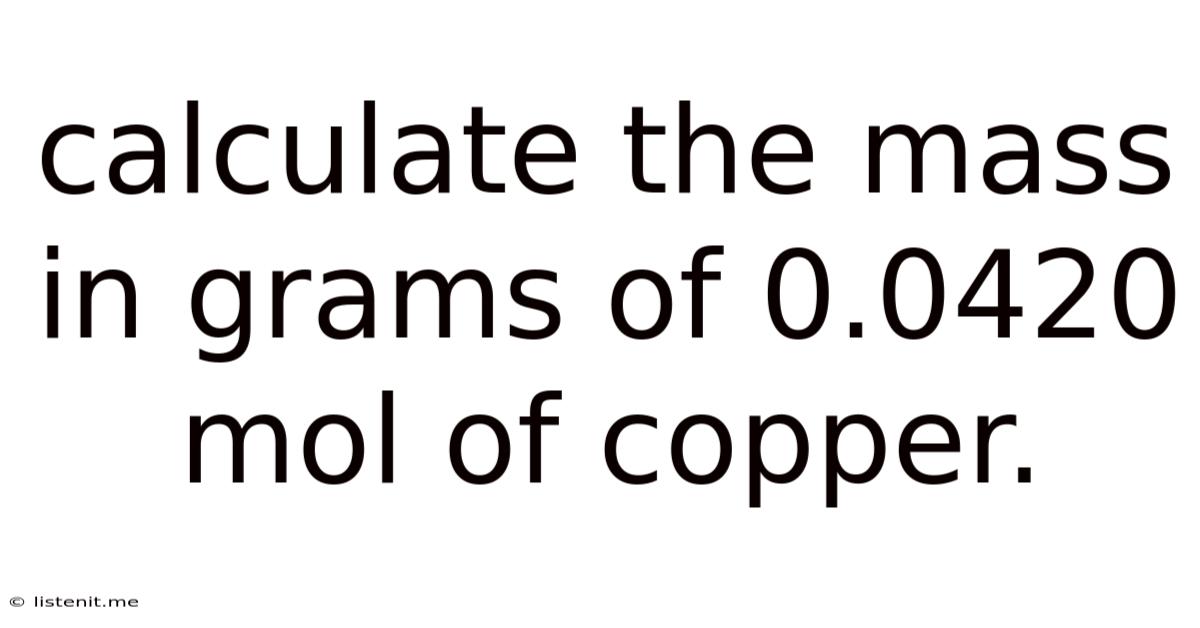
Table of Contents
Calculate the Mass in Grams of 0.0420 mol of Copper: A Comprehensive Guide
Determining the mass of a substance given its number of moles is a fundamental concept in chemistry. This process, often called a mole-to-mass conversion, relies on the molar mass of the element or compound in question. This article will guide you through the step-by-step calculation of the mass of 0.0420 mol of copper, explaining the underlying principles and providing practical tips for similar calculations.
Understanding Moles and Molar Mass
Before diving into the calculation, let's clarify the key concepts involved:
Moles (mol)
A mole is the fundamental unit of measurement in chemistry that represents a specific number of particles (atoms, molecules, ions, etc.). This number is known as Avogadro's number, approximately 6.022 x 10<sup>23</sup>. One mole of any substance contains Avogadro's number of particles.
Molar Mass (g/mol)
Molar mass represents the mass of one mole of a substance. It's expressed in grams per mole (g/mol). For elements, the molar mass is numerically equal to the atomic weight found on the periodic table. For compounds, it's the sum of the molar masses of all the atoms in the chemical formula.
Calculating the Mass of Copper
Now, let's tackle the problem: calculating the mass in grams of 0.0420 mol of copper (Cu).
Step 1: Find the Molar Mass of Copper
Consult the periodic table to find the atomic weight of copper. You'll find that copper (Cu) has an atomic weight of approximately 63.55 g/mol. This means one mole of copper has a mass of 63.55 grams.
Step 2: Apply the Mole-to-Mass Conversion Formula
The formula for converting moles to mass is:
Mass (g) = Moles (mol) x Molar Mass (g/mol)
Step 3: Substitute the Values and Calculate
Substitute the known values into the formula:
Mass (g) = 0.0420 mol x 63.55 g/mol
Mass (g) = 2.6691 g
Therefore, the mass of 0.0420 mol of copper is approximately 2.67 grams.
Significance of Significant Figures
In scientific calculations, it's crucial to pay attention to significant figures. Significant figures represent the digits in a number that carry meaning contributing to its precision. The number of significant figures in the final answer should reflect the least precise measurement used in the calculation.
In our example:
- 0.0420 mol has three significant figures.
- 63.55 g/mol has four significant figures.
Therefore, the final answer should have three significant figures, rounding 2.6691 g to 2.67 g.
Practical Applications and Further Exploration
The ability to convert between moles and mass is crucial in various chemical calculations and applications, including:
- Stoichiometry: Determining the amounts of reactants and products in chemical reactions. Understanding mole-to-mass conversions is fundamental for solving stoichiometry problems, which are essential for predicting the yield of a chemical reaction and optimizing chemical processes.
- Solution Preparation: Preparing solutions with specific concentrations requires precise calculations involving moles and mass. This is vital in analytical chemistry, biochemistry, and pharmaceutical sciences where accurate solutions are essential for experiments and treatments.
- Quantitative Analysis: Analytical techniques such as titration often involve calculations based on the moles and mass of substances involved. This aspect is critical in environmental monitoring, food safety, and quality control.
Advanced Concepts and Related Calculations
Let's explore some related concepts and calculations that build upon the mole-to-mass conversion:
Mass-to-Moles Conversion
The reverse calculation—converting mass to moles—is equally important. The formula is:
Moles (mol) = Mass (g) / Molar Mass (g/mol)
For instance, if you have 5.00 g of copper, you can calculate the number of moles:
Moles (mol) = 5.00 g / 63.55 g/mol = 0.0787 mol
Calculations Involving Compounds
The same principles apply to compounds. To calculate the mass of a given number of moles of a compound, you need to determine the molar mass of the compound first by adding up the molar masses of all its constituent atoms. For example, to find the mass of 0.25 moles of water (H₂O), you would first calculate the molar mass of water (18.02 g/mol) and then use the mole-to-mass formula.
Percentage Composition
Percentage composition describes the mass percentage of each element in a compound. You can calculate this using the molar mass of each element and the compound's molar mass. This knowledge allows for analysis of a compound's elemental makeup.
Empirical and Molecular Formulas
Determining empirical and molecular formulas from experimental data often involves mole-to-mass conversions. The empirical formula represents the simplest whole-number ratio of atoms in a compound, while the molecular formula indicates the actual number of atoms in a molecule.
Conclusion
Mastering mole-to-mass conversions is essential for success in chemistry. This comprehensive guide has provided a detailed walkthrough of calculating the mass of 0.0420 mol of copper, emphasizing the importance of understanding moles, molar mass, and significant figures. By understanding these fundamental concepts and applying the formulas correctly, you can confidently tackle various chemical calculations and unlock a deeper understanding of the quantitative aspects of chemistry. Remember to always consult the periodic table for accurate atomic weights and pay close attention to significant figures for precise results. The ability to perform these calculations is a cornerstone of success in chemistry and related scientific fields. This knowledge is crucial not only for academics but also in various professional contexts where chemical calculations and analyses are vital.
Latest Posts
Latest Posts
-
Probability Density Function Of Chi Square Distribution
May 12, 2025
-
Do Isotopes Have The Same Chemical Properties
May 12, 2025
-
Equation Of Circle With Centre At Origin
May 12, 2025
-
How Many Protons Neutrons And Electrons Does Copper Have
May 12, 2025
-
Balanced Equation For The Combustion Of Hexane
May 12, 2025
Related Post
Thank you for visiting our website which covers about Calculate The Mass In Grams Of 0.0420 Mol Of Copper. . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.