Balanced Equation For The Combustion Of Hexane
listenit
May 12, 2025 · 5 min read
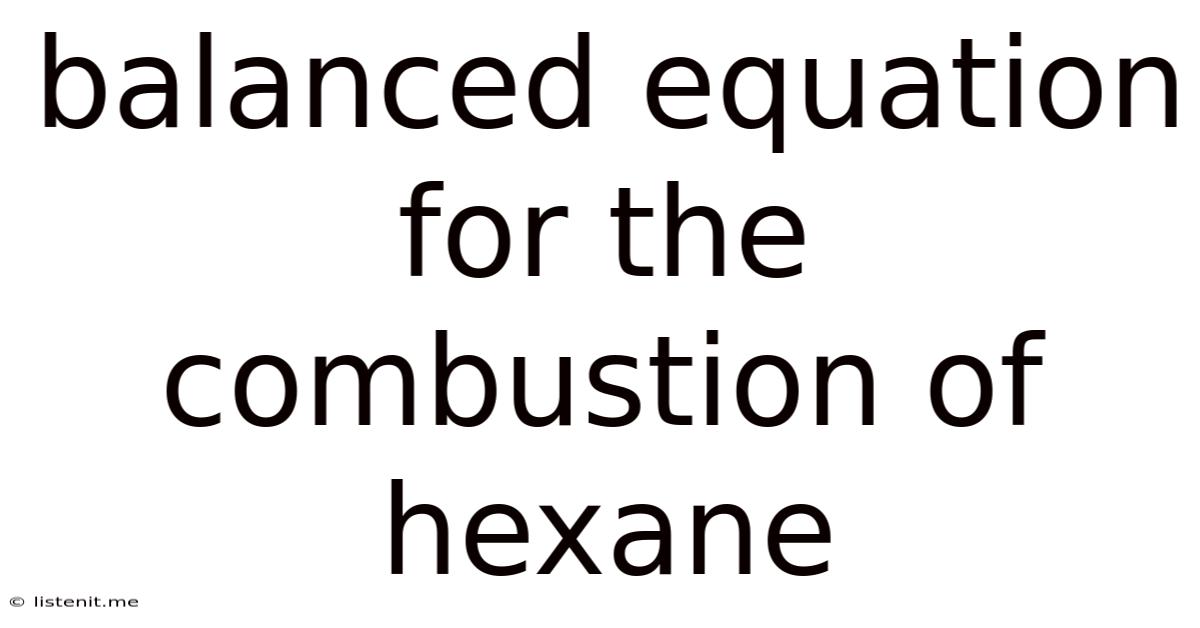
Table of Contents
The Balanced Equation for the Combustion of Hexane: A Comprehensive Guide
The combustion of hexane, a common alkane found in gasoline, is a crucial chemical process with significant implications in various fields, from energy production to environmental science. Understanding the balanced chemical equation for this reaction is fundamental to comprehending its stoichiometry, energy release, and environmental impact. This comprehensive guide will delve into the balanced equation, explore the reaction mechanism, and discuss the practical applications and environmental considerations surrounding hexane combustion.
Understanding the Combustion Reaction
Combustion, in its simplest form, is a rapid, exothermic redox reaction between a fuel and an oxidant, usually oxygen. In the case of hexane, a hydrocarbon, the combustion process involves the breaking of carbon-hydrogen and carbon-carbon bonds and the formation of new bonds with oxygen to produce carbon dioxide and water. The energy released during this bond rearrangement manifests as heat and light.
The Basic Unbalanced Equation
Before achieving the balanced equation, let's look at the basic, unbalanced chemical equation for the complete combustion of hexane:
C₆H₁₄ + O₂ → CO₂ + H₂O
This equation shows the reactants (hexane and oxygen) and the products (carbon dioxide and water). However, it's crucial to understand that this equation is not balanced; the number of atoms of each element is not equal on both sides of the equation. Balancing the equation is essential for accurately representing the stoichiometry of the reaction.
Balancing the Equation: A Step-by-Step Approach
Balancing a chemical equation involves adjusting the stoichiometric coefficients (the numbers in front of each chemical formula) so that the number of atoms of each element is the same on both the reactant and product sides. Here's a step-by-step approach to balancing the combustion of hexane:
-
Start with Carbon: There are six carbon atoms in one molecule of hexane (C₆H₁₄). Therefore, we need six molecules of carbon dioxide (CO₂) on the product side to balance the carbon atoms:
C₆H₁₄ + O₂ → 6CO₂ + H₂O -
Balance Hydrogen: Next, we balance the hydrogen atoms. There are fourteen hydrogen atoms in one molecule of hexane. Each water molecule (H₂O) contains two hydrogen atoms, so we need seven water molecules to balance the hydrogen:
C₆H₁₄ + O₂ → 6CO₂ + 7H₂O -
Balance Oxygen: Finally, we balance the oxygen atoms. On the product side, we have twelve oxygen atoms from six CO₂ molecules (6 x 2 = 12) and seven oxygen atoms from seven H₂O molecules (7 x 1 = 7), totaling nineteen oxygen atoms. Therefore, we need 19/2 molecules of O₂ on the reactant side:
C₆H₁₄ + 19/2O₂ → 6CO₂ + 7H₂O -
Simplify (Optional): While the equation above is balanced, it's conventionally preferable to have whole-number coefficients. To achieve this, we multiply the entire equation by two:
2C₆H₁₄ + 19O₂ → 12CO₂ + 14H₂O
This is the fully balanced chemical equation for the complete combustion of hexane. This equation shows that two molecules of hexane react with nineteen molecules of oxygen to produce twelve molecules of carbon dioxide and fourteen molecules of water.
Reaction Mechanism: A Deeper Dive
While the balanced equation provides an overall representation of the reaction, it doesn't reveal the detailed steps involved in the combustion process. The actual mechanism is complex and involves several intermediate steps, including radical chain reactions. These reactions involve the formation and breaking of free radicals, highly reactive species with unpaired electrons.
The process typically begins with the initiation step, where heat or a spark breaks down oxygen molecules into highly reactive oxygen radicals. These radicals then react with hexane molecules, initiating a chain reaction. The chain propagation steps involve the sequential addition of oxygen radicals to the hexane molecule, breaking carbon-hydrogen and carbon-carbon bonds and forming various intermediate radicals. Finally, the chain termination steps involve the recombination of radicals to form stable products, primarily carbon dioxide and water.
Practical Applications and Significance
The combustion of hexane, along with other hydrocarbons, is central to numerous applications:
-
Energy Production: Hexane is a component of gasoline and other fuels, providing a significant source of energy for transportation and power generation. The heat released during its combustion drives internal combustion engines in vehicles and power turbines in power plants.
-
Industrial Processes: Hexane combustion finds use in various industrial processes where controlled heat is required. This might include heating and drying operations.
-
Laboratory Applications: The controlled combustion of hexane in laboratory settings provides a calibrated heat source for certain experiments and calibrations.
Environmental Considerations
While hexane combustion provides energy, it also has environmental implications:
-
Greenhouse Gas Emissions: The combustion of hexane produces carbon dioxide (CO₂), a major greenhouse gas contributing to global warming and climate change.
-
Air Pollution: Incomplete combustion of hexane can lead to the formation of harmful pollutants like carbon monoxide (CO), particulate matter, and nitrogen oxides (NOx), impacting air quality and human health.
-
Resource Depletion: The reliance on hexane as a fuel contributes to the depletion of fossil fuel resources.
Mitigating Environmental Impact
Several strategies can mitigate the environmental impact of hexane combustion:
-
Improving Combustion Efficiency: Optimizing combustion processes to ensure complete burning of hexane minimizes the formation of harmful pollutants.
-
Carbon Capture and Storage (CCS): Implementing CCS technologies captures CO₂ emissions from combustion processes and stores them underground, preventing their release into the atmosphere.
-
Transition to Renewable Energy: Shifting towards renewable energy sources like solar, wind, and hydro reduces the dependence on fossil fuels and mitigates greenhouse gas emissions.
-
Developing Alternative Fuels: Research and development of alternative fuels, such as biofuels or hydrogen, offer potential alternatives to reduce reliance on fossil fuel-based hydrocarbons like hexane.
Conclusion
The balanced equation for the combustion of hexane, 2C₆H₁₄ + 19O₂ → 12CO₂ + 14H₂O, is a fundamental representation of a significant chemical reaction. Understanding this equation is crucial for comprehending the stoichiometry, energy release, and environmental implications of hexane combustion. While hexane combustion serves essential energy needs, its environmental impact necessitates continuous efforts towards improving combustion efficiency, implementing carbon capture technologies, and transitioning towards cleaner and more sustainable energy sources. This comprehensive understanding enables informed decision-making and promotes responsible energy practices to balance the benefits of hexane combustion with the need for environmental protection.
Latest Posts
Latest Posts
-
What Gas Is Released During Photosynthesis
May 12, 2025
-
What Unit Is Used To Measure Air Pressure
May 12, 2025
-
What Affects The Rate Of Diffusion
May 12, 2025
-
How Do Elements Heavier Than Iron Form
May 12, 2025
-
Is The Square Root Of 5 Rational Or Irrational
May 12, 2025
Related Post
Thank you for visiting our website which covers about Balanced Equation For The Combustion Of Hexane . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.