Boiling Water Is A Physical Or Chemical Change
listenit
Mar 22, 2025 · 5 min read
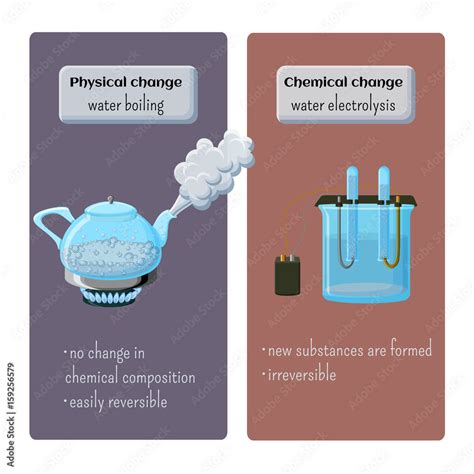
Table of Contents
Boiling Water: A Physical Change Explained
Is boiling water a physical or chemical change? This seemingly simple question opens the door to a fascinating exploration of matter, its properties, and the fundamental differences between physical and chemical transformations. While the answer might appear obvious at first glance, a deeper understanding requires examining the molecular level and the defining characteristics of each change type. This comprehensive guide will delve into the intricacies of boiling water, definitively establishing its classification and dispelling any lingering misconceptions.
Understanding Physical and Chemical Changes
Before classifying the boiling of water, we need a clear definition of both physical and chemical changes. These two categories encompass all transformations matter can undergo.
Physical Changes: A Matter of Form, Not Substance
A physical change alters the form or appearance of a substance but does not change its chemical composition. Think of it as rearranging the furniture in a room—the room's contents remain the same, just organized differently. Examples of physical changes include:
- Changes in state: Melting ice, freezing water, boiling water, condensing steam—all these involve a change in the physical state (solid, liquid, gas) but not the chemical makeup of the water (H₂O).
- Changes in shape: Cutting paper, bending a metal rod, crushing a can. The material's composition remains unchanged.
- Dissolving: Salt dissolving in water is a physical change because the salt molecules are dispersed in the water but still retain their chemical identity. They can be recovered through evaporation.
Chemical Changes: Breaking and Making Bonds
A chemical change, also known as a chemical reaction, involves a change in the chemical composition of a substance. New substances with different properties are formed. This is akin to remodeling the room, completely changing its structure and contents. Key indicators of chemical changes include:
- Formation of a gas: Bubbles, fizzing, or the release of a gas often signal a chemical reaction.
- Formation of a precipitate: A solid forming from a solution.
- Color change: A significant and unexpected color change can suggest a chemical reaction.
- Temperature change (exothermic or endothermic): Chemical reactions either release heat (exothermic) or absorb heat (endothermic).
- Irreversibility: Many chemical changes are difficult or impossible to reverse without further chemical reactions.
The Boiling of Water: A Detailed Examination
Now, let's analyze the process of boiling water through the lens of physical and chemical changes. When water boils, it transitions from a liquid state to a gaseous state, forming steam. This phase transition involves:
- Increased kinetic energy: Heat energy applied to the water increases the kinetic energy of its molecules. The molecules move faster and faster.
- Breaking intermolecular forces: Water molecules are held together by relatively strong intermolecular forces (hydrogen bonds). As the kinetic energy increases, these forces are overcome.
- Phase transition: The molecules gain enough energy to escape the liquid phase and enter the gaseous phase (steam).
Crucially, the chemical composition of water remains unchanged. Each water molecule (H₂O) in the steam is identical to those in the liquid water. There is no breaking or forming of chemical bonds within the water molecules themselves. The process only involves altering the arrangement and energy of the existing molecules.
Distinguishing Boiling from Decomposition
It's important to differentiate boiling from a chemical decomposition. Decomposition is a chemical change where a single compound breaks down into two or more simpler substances. For example, the decomposition of water into hydrogen and oxygen requires a significant input of energy (electrolysis) and results in the formation of entirely new substances with different chemical properties. This is drastically different from simply boiling water.
Boiling water is a physical change because the chemical identity of the water remains constant throughout the process. Only the physical state changes from liquid to gas. The molecules of water are simply moving further apart, not changing their fundamental composition.
Misconceptions about Boiling Water
Some might argue that the production of steam involves a chemical reaction because of the energy input. However, the energy is used to overcome intermolecular forces, not to break chemical bonds within the water molecule itself. The energy is a catalyst for a physical change, not a participant in a chemical reaction.
Another common misconception is that the formation of bubbles during boiling signifies a chemical change. However, the bubbles are primarily composed of water vapor (steam), not new chemical substances. They are simply a visual manifestation of the phase transition.
Practical Applications and Real-World Examples
Understanding the physical nature of boiling water has numerous practical applications:
- Cooking: Boiling water is essential in cooking various foods, relying on the transfer of heat to cook ingredients, not a chemical change in the water itself.
- Steam generation: The steam produced from boiling water is used for various purposes, including electricity generation and sterilization.
- Distillation: Boiling and condensing water is a crucial step in the purification process of distillation. The process relies on the difference in boiling points to separate substances, again highlighting the physical nature of the boiling process.
- Weather patterns: The evaporation and condensation of water are key components of weather patterns, demonstrating the importance of the phase transitions in the water cycle.
The ability to boil water efficiently and safely has been paramount to human progress, furthering technological advancements and our understanding of the world around us.
Conclusion: Boiling Water Remains a Physical Change
In conclusion, boiling water is unequivocally a physical change. The process only involves a change in the state of water from liquid to gas, without altering its chemical composition. The molecules of water remain intact; only their arrangement and energy levels change. While energy input is necessary to initiate the boiling process, this energy is used to overcome intermolecular forces, not to break chemical bonds. Understanding this fundamental distinction is crucial for comprehending the nature of matter and its transformations. The seemingly simple act of boiling water reveals the deeper, fascinating world of physical and chemical changes, a cornerstone of chemistry and material science. By clarifying this fundamental concept, we establish a solid foundation for further scientific exploration and discovery. This understanding opens up possibilities for innovation in various fields, from culinary arts to industrial processes, all underpinned by the fundamental physics of boiling water.
Latest Posts
Latest Posts
-
What Percent Of 20 Is 6
Mar 23, 2025
-
Whats The Square Root Of 1 4
Mar 23, 2025
-
3 5 As A Decimal And Percent
Mar 23, 2025
-
When Nacl Is Dissolved In Water
Mar 23, 2025
-
Boiling Of Water Is A Physical Change
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Boiling Water Is A Physical Or Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
