Atoms That Gain Or Lose Electrons Are Called
listenit
Apr 01, 2025 · 6 min read
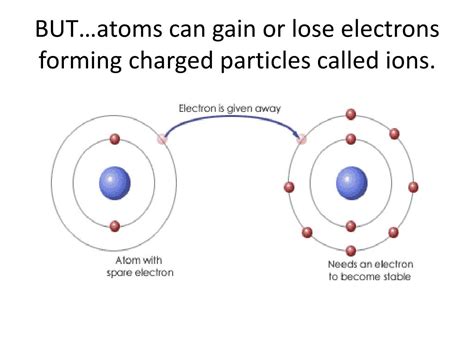
Table of Contents
Atoms That Gain or Lose Electrons Are Called Ions: A Deep Dive into Ionic Bonding and Its Implications
Atoms are the fundamental building blocks of matter, but their behavior is far from simple. One crucial aspect of atomic behavior is their tendency to gain or lose electrons, a process that fundamentally alters their properties and leads to the formation of chemical bonds. Atoms that undergo this electron transfer are called ions, and understanding their characteristics is key to comprehending a vast array of chemical and physical phenomena. This article will delve into the fascinating world of ions, exploring their formation, properties, and significance in various contexts.
Understanding the Basics: Protons, Electrons, and the Quest for Stability
Before diving into the specifics of ions, let's briefly review the fundamental components of an atom: protons, neutrons, and electrons. Protons carry a positive charge and reside in the atom's nucleus, along with neutrons which are electrically neutral. Electrons, carrying a negative charge, orbit the nucleus in electron shells or energy levels.
The number of protons in an atom's nucleus determines its atomic number and defines the element. For instance, an atom with one proton is hydrogen, while an atom with six protons is carbon. In a neutral atom, the number of protons equals the number of electrons, resulting in a net charge of zero.
However, atoms often strive for stability, typically by achieving a full outermost electron shell (also known as the valence shell). This stable configuration, often resembling the electron arrangement of noble gases, is a driving force behind many chemical reactions. Atoms can achieve this stable state by either gaining or losing electrons, transforming them into ions.
Ions: A Charged Affair
When an atom gains or loses electrons, it acquires a net electrical charge, becoming an ion. Ions are classified into two main categories:
Cations: Positively Charged Ions
Cations are formed when an atom loses one or more electrons. Because electrons carry a negative charge, their loss leaves the atom with more protons than electrons, resulting in a net positive charge. The magnitude of the positive charge is indicated by a superscript following the element's symbol. For example, a sodium atom (Na) readily loses one electron to become a sodium cation (Na⁺). Similarly, magnesium (Mg) can lose two electrons to form Mg²⁺. The tendency of an atom to lose electrons is often associated with elements on the left side of the periodic table, particularly metals.
Key Characteristics of Cations:
- Positive charge: Due to electron loss.
- Smaller size than the parent atom: Loss of electrons reduces electron-electron repulsion, leading to a smaller ionic radius.
- Often found in ionic compounds: Cations readily bond with anions to form stable ionic compounds.
- Important roles in biological systems: Many essential biological processes rely on the presence of specific cations, like sodium (Na⁺), potassium (K⁺), calcium (Ca²⁺), and magnesium (Mg²⁺) ions.
Anions: Negatively Charged Ions
Anions are formed when an atom gains one or more electrons. This addition of negatively charged electrons results in more electrons than protons, creating a net negative charge. Similar to cations, the magnitude of the negative charge is represented by a superscript. For example, a chlorine atom (Cl) readily gains one electron to become a chloride anion (Cl⁻). Oxygen (O) can gain two electrons to form the oxide anion (O²⁻). Atoms with a high electron affinity, typically non-metals located on the right side of the periodic table, tend to form anions.
Key Characteristics of Anions:
- Negative charge: Due to electron gain.
- Larger size than the parent atom: The addition of electrons increases electron-electron repulsion, leading to a larger ionic radius.
- Often found in ionic compounds: Anions bond with cations to form stable ionic compounds.
- Important roles in biological systems: Various anions, like chloride (Cl⁻), phosphate (PO₄³⁻), and sulfate (SO₄²⁻), play crucial roles in biological systems.
Ionic Bonding: The Electrostatic Attraction
The formation of ions is intimately connected with ionic bonding. Ionic bonds are electrostatic forces of attraction between oppositely charged ions. The strong electrostatic attraction between a cation and an anion holds them together, forming a stable ionic compound.
Example: Consider the formation of sodium chloride (NaCl), common table salt. A sodium atom (Na) loses one electron to become a sodium cation (Na⁺), while a chlorine atom (Cl) gains that electron to become a chloride anion (Cl⁻). The electrostatic attraction between the positively charged Na⁺ and the negatively charged Cl⁻ ions forms the ionic bond that holds the sodium chloride crystal lattice together.
The strength of an ionic bond depends on several factors:
- Magnitude of charges: Higher charges lead to stronger attraction.
- Distance between ions: Shorter distances result in stronger attraction.
- Size of ions: Smaller ions result in stronger attraction.
Properties of Ionic Compounds
Ionic compounds, formed through ionic bonding, exhibit distinct properties:
- High melting and boiling points: The strong electrostatic forces between ions require significant energy to overcome, resulting in high melting and boiling points.
- Crystalline structure: Ions are arranged in a regular, three-dimensional lattice structure.
- Brittle nature: A slight shift in the crystal lattice can lead to repulsion between like charges, causing the crystal to fracture.
- Solubility in water: Many ionic compounds are soluble in water, as water molecules can effectively surround and solvate the ions.
- Conductivity in molten or aqueous states: When molten or dissolved in water, ions are free to move, allowing the compound to conduct electricity.
Significance of Ions in Various Fields
The importance of ions extends far beyond the realm of chemistry. They play pivotal roles in various fields:
Biology:
Ions are essential for numerous biological processes. For instance, nerve impulses rely on the movement of sodium (Na⁺) and potassium (K⁺) ions across cell membranes. Calcium (Ca²⁺) ions are crucial for muscle contraction and bone formation. Chloride (Cl⁻) ions contribute to maintaining osmotic balance. Phosphate (PO₄³⁻) ions are vital components of DNA and ATP (adenosine triphosphate), the energy currency of cells.
Medicine:
Many medical treatments utilize ions. Intravenous fluids often contain specific ions to maintain electrolyte balance. Medical imaging techniques, such as MRI (magnetic resonance imaging), exploit the properties of ions in the body. Electrolyte imbalances can have severe health consequences, highlighting the importance of maintaining proper ion levels in the body.
Industry:
Ions find widespread applications in various industries. Electroplating uses ionic solutions to deposit metal coatings on surfaces. Batteries rely on the movement of ions to generate electrical energy. Water treatment processes often involve ion exchange to remove impurities.
Environmental Science:
Ions play a significant role in environmental processes. Acid rain, caused by the release of acidic ions like sulfate (SO₄²⁻) and nitrate (NO₃⁻) into the atmosphere, poses significant environmental threats. The presence and concentration of ions in water bodies are vital indicators of water quality.
Conclusion: The Ubiquitous Nature of Ions
Atoms that gain or lose electrons are called ions, and their formation profoundly impacts the properties of matter. The electrostatic attraction between cations and anions leads to ionic bonding, which underlies the formation of a wide array of compounds with unique characteristics. Ions play crucial roles in biological systems, medical treatments, industrial processes, and environmental phenomena, underscoring their ubiquitous nature and fundamental importance in the world around us. A deeper understanding of ions is essential for advancements in various scientific and technological fields.
Latest Posts
Latest Posts
-
What Is The Bottom Of A Wave Called
Apr 02, 2025
-
Greatest Common Factor Of 18 And 30
Apr 02, 2025
-
Miles Per Hour To Meters Per Minute
Apr 02, 2025
-
Has A Definite Volume And Shape
Apr 02, 2025
-
What Is 3 Out Of 25 As A Percentage
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Atoms That Gain Or Lose Electrons Are Called . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
