Are Temperature And Pressure Directly Proportional
listenit
Mar 19, 2025 · 5 min read
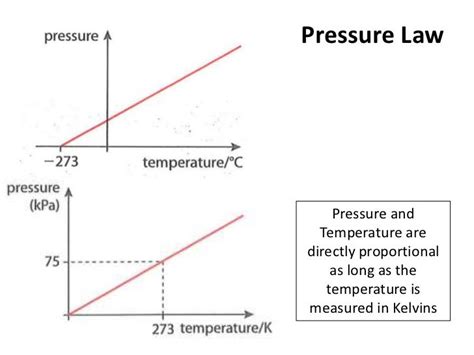
Table of Contents
Are Temperature and Pressure Directly Proportional? Exploring the Relationship in Ideal and Real Gases
The relationship between temperature and pressure is a fundamental concept in physics and chemistry, particularly within the context of gases. A common initial understanding suggests a direct proportionality: as temperature increases, pressure increases, and vice versa. However, the reality is more nuanced, depending heavily on the conditions and the nature of the gas itself. This article will delve deep into the relationship between temperature and pressure, exploring the ideal gas law, deviations in real gases, and the various factors influencing this interaction.
The Ideal Gas Law: A Foundation for Understanding
The ideal gas law serves as a foundational model for understanding the relationship between temperature, pressure, volume, and the amount of gas present. It's expressed mathematically as:
PV = nRT
Where:
- P represents pressure
- V represents volume
- n represents the number of moles of gas
- R is the ideal gas constant
- T represents temperature (in Kelvin)
This equation reveals a direct proportionality between pressure (P) and temperature (T) when the volume (V) and the number of moles (n) are held constant. In other words, if you double the absolute temperature, keeping the volume and amount of gas constant, you will double the pressure. This is often referred to as Gay-Lussac's Law.
Important Note: The temperature in the ideal gas law must be expressed in Kelvin. Using Celsius or Fahrenheit will lead to inaccurate results because these scales have arbitrary zero points. Kelvin starts at absolute zero, the theoretical point where all molecular motion ceases.
Understanding Direct Proportionality in the Ideal Gas Law
The direct proportionality between temperature and pressure in the ideal gas law stems from the kinetic theory of gases. This theory posits that gas particles are in constant, random motion. Their collisions with the container walls exert pressure. Increasing the temperature increases the average kinetic energy of these particles, meaning they move faster and collide with the walls more frequently and with greater force. This leads to a higher pressure.
Visualizing the Direct Proportionality
Imagine a sealed container filled with a gas. If you increase the temperature, the gas particles will gain kinetic energy, moving faster. This increased kinetic energy translates to more frequent and forceful collisions with the container walls, resulting in a higher pressure. Conversely, decreasing the temperature slows down the particles, leading to fewer and less forceful collisions, and therefore lower pressure.
Limitations of the Ideal Gas Law
It's crucial to remember that the ideal gas law is a simplification. It assumes that gas particles have negligible volume and that there are no intermolecular forces between them. These assumptions hold true only under specific conditions – typically at low pressures and high temperatures.
Deviations from Ideality: Real Gases and the Compressibility Factor
Real gases deviate from the ideal gas law, particularly at high pressures and low temperatures. At high pressures, the volume of the gas particles themselves becomes significant compared to the total volume, invalidating the assumption of negligible particle volume. At low temperatures, intermolecular forces become more prominent, leading to attractions between gas particles that reduce the pressure exerted on the container walls.
The compressibility factor (Z) is a measure of how much a real gas deviates from ideal behavior. It is defined as:
Z = PV/nRT
For an ideal gas, Z = 1. For real gases, Z can be greater than or less than 1, depending on the pressure and temperature. A Z value greater than 1 indicates that the gas is more compressible than an ideal gas (due to significant intermolecular forces), while a Z value less than 1 indicates that it's less compressible (due to significant particle volume).
Factors Affecting the Temperature-Pressure Relationship in Real Gases
Several factors beyond temperature itself influence the pressure of a real gas:
-
Intermolecular Forces: Attractive forces between gas molecules (like van der Waals forces) reduce the pressure exerted on the container walls. At low temperatures, these forces become more significant.
-
Molecular Volume: At high pressures, the volume occupied by the gas molecules themselves becomes a substantial fraction of the total volume, causing deviations from ideal behavior.
-
Type of Gas: Different gases have different intermolecular forces and molecular sizes, leading to varying degrees of deviation from ideal behavior. For instance, polar molecules exhibit stronger intermolecular forces than non-polar molecules.
-
Critical Temperature and Pressure: Every gas has a critical temperature above which it cannot be liquefied, no matter how high the pressure. Near the critical temperature and pressure, deviations from the ideal gas law are significant.
Beyond Gay-Lussac's Law: More Complex Scenarios
While Gay-Lussac's Law describes the direct proportionality under constant volume, more complex scenarios exist where the volume isn't constant. For instance, consider a gas in a container with a movable piston. Heating the gas will not only increase the pressure but also cause the volume to expand, thus complicating the simple direct proportionality. The ideal gas law accounts for this, allowing us to analyze the relationships between all four variables simultaneously.
Applications of the Temperature-Pressure Relationship
Understanding the relationship between temperature and pressure has numerous practical applications:
-
Automotive Engines: The pressure and temperature inside an engine cylinder are intricately linked, affecting combustion efficiency and power output.
-
Weather Forecasting: Atmospheric pressure and temperature are key variables used in weather prediction models.
-
Aerospace Engineering: The pressure and temperature changes experienced by aircraft at high altitudes must be carefully considered in the design and operation of aircraft systems.
-
Industrial Processes: Many industrial processes, such as chemical reactions and refrigeration, involve manipulating temperature and pressure to control reaction rates and product yields.
-
Diving: Divers need to understand the relationship between pressure and temperature to account for changes in gas volume at different depths and temperatures.
Conclusion: A Nuanced Relationship
While the ideal gas law suggests a direct proportionality between temperature and pressure at constant volume, real gases exhibit deviations from this ideal behavior, especially at high pressures and low temperatures. The extent of deviation depends on intermolecular forces, molecular volume, and the specific gas in question. Understanding this nuanced relationship is crucial in various fields, from engineering and industrial processes to meteorology and even scuba diving. While Gay-Lussac's Law provides a useful starting point, a comprehensive understanding requires acknowledging the complexities introduced by real-world conditions and the limitations of the ideal gas model. The interplay between temperature and pressure isn't merely a simple direct proportionality; it's a dynamic relationship shaped by multiple interacting factors.
Latest Posts
Latest Posts
-
Where Is A Neutron Located In An Atom
Mar 20, 2025
-
What Is The Subscript In Chemistry
Mar 20, 2025
-
How Many Hydrogen Atoms Are In
Mar 20, 2025
-
What Is 85 As A Fraction
Mar 20, 2025
-
What Is 1 4 Divided By 1 8
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Are Temperature And Pressure Directly Proportional . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
