Where Is A Neutron Located In An Atom
listenit
Mar 20, 2025 · 6 min read
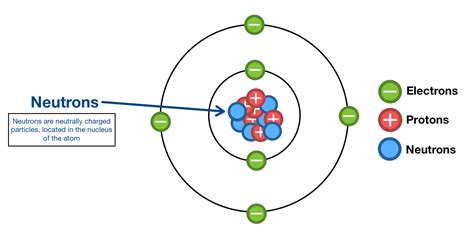
Table of Contents
Where Is a Neutron Located in an Atom? Unraveling the Mysteries of the Nucleus
Understanding the location of a neutron within an atom is fundamental to comprehending the structure of matter itself. This seemingly simple question delves into the fascinating realm of nuclear physics, revealing the intricacies of atomic structure and the forces that govern the subatomic world. This article will explore the precise location of neutrons, the forces holding them in place, and the consequences of their arrangement.
The Atomic Nucleus: The Neutron's Home
The atom, the basic building block of matter, isn't a solid, indivisible sphere as once thought. Instead, it's a complex system composed of a tiny, dense nucleus at its center and a cloud of orbiting electrons. The nucleus, significantly smaller than the atom as a whole, contains two types of particles: protons and neutrons. It's within this nucleus, specifically, that we find the neutron.
Protons, Neutrons, and the Strong Nuclear Force
The nucleus isn't simply a jumble of protons and neutrons. Protons, possessing a positive electrical charge, repel each other strongly due to the electromagnetic force. This repulsive force is enormous and should, theoretically, cause the nucleus to fly apart. However, this doesn't happen thanks to the strong nuclear force, a fundamental force of nature far stronger than the electromagnetic force, but acting only over extremely short distances – within the confines of the nucleus.
The strong nuclear force binds protons and neutrons together, overcoming the electromagnetic repulsion between protons. Neutrons, possessing no net electrical charge (they are neutral), play a crucial role in stabilizing the nucleus. They act as a kind of "glue," effectively spacing the positively charged protons apart and mitigating the repulsive forces.
Precise Location: It's Not a Simple Answer
Defining the exact location of a neutron within the nucleus isn't straightforward. Unlike the planetary model of the atom, which depicts electrons orbiting the nucleus in clearly defined paths, the location of nucleons (protons and neutrons) is described by quantum mechanics. This means their position and momentum cannot be precisely determined simultaneously – a consequence of the Heisenberg Uncertainty Principle.
Instead of a fixed location, we describe the neutron's position in terms of probability. Quantum mechanics predicts the likelihood of finding a neutron within a certain volume of the nucleus. This probability is described by a wave function, a mathematical function that provides the probability amplitude of finding the particle at a given point in space. The square of the wave function's magnitude gives the probability density.
Nuclear Models and Neutron Distribution
Scientists use various nuclear models to approximate the distribution of neutrons within the nucleus. These models, while imperfect, provide valuable insights into nuclear structure. Some key models include:
-
The Liquid Drop Model: This model treats the nucleus as a droplet of incompressible nuclear fluid. Neutrons and protons are distributed relatively uniformly throughout this fluid, with a slightly higher density towards the center. This model is particularly useful for explaining nuclear fission.
-
The Shell Model: This model proposes that nucleons occupy distinct energy levels or shells within the nucleus, similar to the electron shells in an atom. The filling of these shells influences the stability and properties of the nucleus. Neutrons and protons fill these shells independently, but the overall configuration affects the stability. This model explains the existence of "magic numbers" – specific numbers of neutrons or protons that result in exceptionally stable nuclei.
-
The Collective Model: This model combines aspects of the liquid drop and shell models, accounting for both the overall distribution of nucleons and the individual motions of nucleons within the nucleus.
Factors Influencing Neutron Location
Several factors affect the probability distribution of neutrons within the nucleus:
-
Number of neutrons: The total number of neutrons significantly impacts their overall distribution. A greater number of neutrons necessitates a larger nuclear volume to accommodate them.
-
Number of protons: The number of protons influences the distribution due to the electromagnetic repulsion between them. Neutrons help mitigate this repulsion, and their distribution adapts to optimize the overall nuclear stability.
-
Isotopes: Isotopes are atoms of the same element with different numbers of neutrons. Different isotopes of the same element have varying neutron distributions, impacting their stability and properties. For example, carbon-12 (6 protons, 6 neutrons) is stable, whereas carbon-14 (6 protons, 8 neutrons) is radioactive due to the different neutron distributions and subsequent nuclear instability.
-
Nuclear Shape: The shape of the nucleus, which can be spherical, prolate (elongated), or oblate (flattened), affects the neutron distribution. In deformed nuclei, neutrons may be more concentrated in certain regions.
Consequences of Neutron Distribution
The distribution of neutrons within the nucleus has profound implications:
-
Nuclear stability: A balanced distribution of neutrons and protons is crucial for nuclear stability. An imbalance can lead to radioactive decay, where the nucleus emits particles or energy to achieve a more stable configuration.
-
Nuclear reactions: The spatial arrangement of neutrons influences the likelihood of nuclear reactions, such as fission (splitting of the nucleus) and fusion (combining of nuclei). Neutron bombardment, for instance, is a common method used to initiate fission in nuclear reactors.
-
Nuclear properties: Properties like nuclear spin, magnetic moment, and binding energy are directly related to the neutron distribution within the nucleus.
Advanced Concepts: Neutron Density and Halo Nuclei
Understanding the subtleties of neutron location requires delving into more advanced concepts:
-
Neutron Density: Neutron density isn't uniform throughout the nucleus. It can vary significantly, especially in larger nuclei. It’s higher in the central region and often exhibits a skin effect where the density at the surface differs.
-
Neutron Skin: The phenomenon of a neutron skin, where the neutron distribution extends slightly beyond the proton distribution, is observed in certain isotopes, particularly those with a significant neutron excess. This is a critical area of research because the thickness of the neutron skin offers valuable insights into the properties of the strong nuclear force.
-
Neutron Halo Nuclei: Some isotopes exhibit a neutron halo, where a few neutrons are loosely bound and their probability distribution extends far beyond the main nuclear volume. These loosely bound neutrons are highly susceptible to interaction and significantly impact the nucleus's overall properties. Understanding these halo nuclei offers crucial insights into the structure and interactions at the very edge of nuclear matter.
Conclusion: A Probabilistic Picture of the Neutron's Abode
The location of a neutron within an atom is not a simple point in space but rather a probability distribution determined by quantum mechanics and the intricate interplay of nuclear forces. Although we can't pinpoint the precise location of a neutron, sophisticated nuclear models and experimental techniques give us a good understanding of its probable position and how this position contributes to the atom's overall properties. The study of neutron distribution continues to be a vibrant area of research, revealing fundamental insights into the structure of matter and the fundamental forces of nature. This knowledge is crucial for advancements in nuclear energy, nuclear medicine, and our fundamental understanding of the universe. The exploration of neutron distribution and its effects on nuclear properties remains a fertile ground for scientific discovery, pushing the boundaries of our understanding of the atomic world. The ongoing research into nuclear structure, aided by advanced experimental techniques and theoretical models, continues to refine our understanding of the neutron's position and its role in the intricate dance of nuclear forces.
Latest Posts
Latest Posts
-
29 Degrees Celsius Is What In Fahrenheit
Mar 20, 2025
-
How Many Valence Electrons Are In Iodine
Mar 20, 2025
-
During Which Of The Following Phases Does Dna Replication Occur
Mar 20, 2025
-
5 Quarts Is How Many Pints
Mar 20, 2025
-
Graph Of X 2 Y 2 9
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Where Is A Neutron Located In An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
