What Is The Subscript In Chemistry
listenit
Mar 20, 2025 · 6 min read
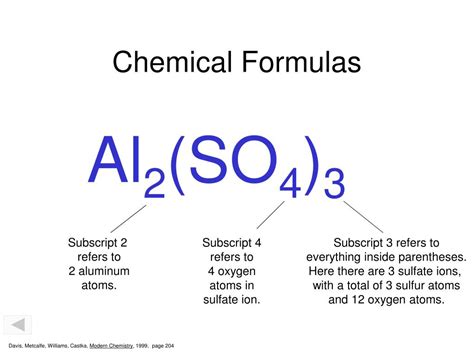
Table of Contents
- What Is The Subscript In Chemistry
- Table of Contents
- What is a Subscript in Chemistry? A Comprehensive Guide
- Understanding the Basics: What Subscripts Represent
- The Significance of Subscripts in Chemical Formulas
- Subscripts vs. Coefficients: A Key Distinction
- Applications of Subscripts Across Chemical Disciplines
- 1. Organic Chemistry:
- 2. Inorganic Chemistry:
- 3. Analytical Chemistry:
- 4. Physical Chemistry:
- 5. Biochemistry:
- Beyond Basic Subscripts: Advanced Concepts
- 1. Polyatomic Ions:
- 2. Hydrates:
- 3. Empirical vs. Molecular Formulas:
- Mastering Subscripts: Practice and Resources
- Conclusion: The Unsung Hero of Chemical Notation
- Latest Posts
- Latest Posts
- Related Post
What is a Subscript in Chemistry? A Comprehensive Guide
Subscripts in chemistry aren't just tiny numbers hanging out below chemical symbols; they're fundamental to understanding the composition and behavior of matter. They represent the quantities of atoms involved in a molecule or compound, unlocking the secrets of chemical formulas and equations. This comprehensive guide will delve deep into the world of subscripts, explaining their meaning, importance, and practical applications within various chemical contexts.
Understanding the Basics: What Subscripts Represent
In chemistry, a subscript is a small number written slightly below and to the right of a chemical symbol. This number indicates the number of atoms of that specific element present in a single molecule or formula unit of a substance. For instance, in the chemical formula for water, H₂O, the subscript "2" after the "H" signifies that each water molecule contains two hydrogen atoms. The absence of a subscript, as with the "O" in H₂O, implies that only one atom of that element is present (in this case, one oxygen atom).
Let's break down some examples to solidify this understanding:
- CO₂ (Carbon Dioxide): The subscript "2" indicates two oxygen atoms bonded to one carbon atom.
- NaCl (Sodium Chloride): No subscripts are explicitly written, implying one atom of sodium (Na) and one atom of chlorine (Cl).
- C₆H₁₂O₆ (Glucose): The subscripts represent six carbon atoms, twelve hydrogen atoms, and six oxygen atoms. This detailed representation is crucial for understanding the structure and properties of glucose.
- H₂SO₄ (Sulfuric Acid): Two hydrogen atoms, one sulfur atom, and four oxygen atoms make up a single molecule of sulfuric acid. The subscripts precisely define the stoichiometry (relative quantities of reactants and products) of this crucial chemical.
The Significance of Subscripts in Chemical Formulas
Subscripts are not mere decorations in chemical formulas; they are critical components that convey crucial information:
- Molecular Composition: They precisely define the number and type of atoms comprising a molecule. This is essential for distinguishing between different compounds that might contain the same elements but in different ratios (e.g., carbon monoxide, CO, versus carbon dioxide, CO₂).
- Molecular Mass Calculation: Knowing the number of atoms of each element allows accurate calculation of the molecular mass (or molar mass) of the substance. This is done by multiplying the number of atoms of each element by its atomic mass and then summing the results. This is fundamental to various stoichiometric calculations.
- Chemical Reactions and Equations: Subscripts play a crucial role in balancing chemical equations, ensuring that the number of atoms of each element is conserved throughout the reaction. This adheres to the fundamental law of conservation of mass.
- Understanding Chemical Properties: The precise arrangement and number of atoms defined by subscripts influence the physical and chemical properties of a substance. For example, the different subscripts in CO and CO₂ lead to vastly different properties – one is a poisonous gas, while the other is a crucial component of our atmosphere.
Subscripts vs. Coefficients: A Key Distinction
It's crucial to differentiate subscripts from coefficients in chemical equations. While subscripts indicate the number of atoms within a molecule, coefficients indicate the number of molecules or moles participating in a chemical reaction.
Consider the balanced chemical equation for the combustion of methane:
CH₄ + 2O₂ → CO₂ + 2H₂O
- Subscripts: The subscripts (4 in CH₄, 2 in O₂, 2 in H₂O) define the composition of each molecule.
- Coefficients: The coefficients (1 in front of CH₄, 2 in front of O₂, 1 in front of CO₂, 2 in front of H₂O) specify the relative number of molecules involved in the reaction. They are essential for ensuring that the equation is balanced, meaning the number of atoms of each element is the same on both sides of the arrow.
Applications of Subscripts Across Chemical Disciplines
Subscripts find widespread application across various branches of chemistry:
1. Organic Chemistry:
Subscripts are essential for representing the complex structures of organic molecules, including hydrocarbons, carbohydrates, proteins, and nucleic acids. The detailed information provided by subscripts allows for accurate representation of the intricate bonding and arrangement of atoms within these large molecules. The complexity and diversity of organic molecules necessitates the precise notation of subscripts.
2. Inorganic Chemistry:
Inorganic chemistry involves the study of compounds that don't primarily contain carbon-hydrogen bonds. Subscripts are crucial for representing the composition of ionic compounds, coordination complexes, and various inorganic materials, defining the stoichiometry of these substances and their chemical behavior.
3. Analytical Chemistry:
Subscripts are fundamental to quantitative analysis in chemistry. They are used in stoichiometric calculations, titrations, and other analytical techniques to determine the concentration and amount of substances in a sample. Accurate subscripts ensure precise and reliable analytical results.
4. Physical Chemistry:
In physical chemistry, subscripts are used in various equations and calculations related to thermodynamics, kinetics, and quantum mechanics. The precise representation of molecular composition is essential for accurate modeling and prediction of chemical behavior.
5. Biochemistry:
Biochemistry deals with the chemical processes occurring within and relating to living organisms. Subscripts are critical for representing the composition of biomolecules such as proteins, carbohydrates, and nucleic acids. The detailed information provided helps to understand their structure, function, and interactions.
Beyond Basic Subscripts: Advanced Concepts
While the basic concept of subscripts is straightforward, several more complex scenarios require deeper understanding:
1. Polyatomic Ions:
Subscripts are used within parentheses to denote multiple polyatomic ions in a formula. For instance, in the formula for calcium phosphate, Ca₃(PO₄)₂, the subscript "2" outside the parenthesis signifies two phosphate (PO₄)³⁻ ions.
2. Hydrates:
Hydrates are compounds containing water molecules incorporated into their crystal structure. Subscripts are used to indicate the number of water molecules associated with each formula unit. For example, CuSO₄·5H₂O (copper(II) sulfate pentahydrate) indicates five water molecules per copper(II) sulfate unit.
3. Empirical vs. Molecular Formulas:
Empirical formulas show the simplest whole-number ratio of atoms in a compound, while molecular formulas represent the actual number of atoms in a molecule. Subscripts differ between these two formulas. For instance, the empirical formula of glucose is CH₂O, while the molecular formula is C₆H₁₂O₆. Both formulas use subscripts, but they express different levels of detail.
Mastering Subscripts: Practice and Resources
Understanding subscripts is crucial for proficiency in chemistry. Regular practice with writing and interpreting chemical formulas is essential. Plenty of online resources, textbooks, and educational websites provide exercises and examples to help you master this fundamental concept. Focusing on the distinction between subscripts and coefficients, and practicing balancing chemical equations, will solidify your understanding.
Conclusion: The Unsung Hero of Chemical Notation
Subscripts, while seemingly small and insignificant, are powerful tools that unlock a vast amount of information about chemical compounds and reactions. They underpin our understanding of molecular composition, stoichiometry, and the properties of matter. Mastering subscripts is crucial for anyone seeking a deeper comprehension of the chemical world, paving the way for success in advanced chemistry studies and related fields. Their precise and concise nature makes them an invaluable component of the language of chemistry. By grasping their significance and applications, you're taking a critical step towards a more profound understanding of the fundamental building blocks of our universe.
Latest Posts
Latest Posts
-
How Many Fl Oz In Half Gallon
Mar 21, 2025
-
What Is 4 Divided By 1 3
Mar 21, 2025
-
What Is 50 F In C
Mar 21, 2025
-
How Do You Convert Joules To Electron Volts
Mar 21, 2025
-
What Is Half Of 1 1 2 Tablespoons
Mar 21, 2025
Related Post
Thank you for visiting our website which covers about What Is The Subscript In Chemistry . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
