Why Chemical Equations Must Be Balanced
listenit
Mar 18, 2025 · 6 min read
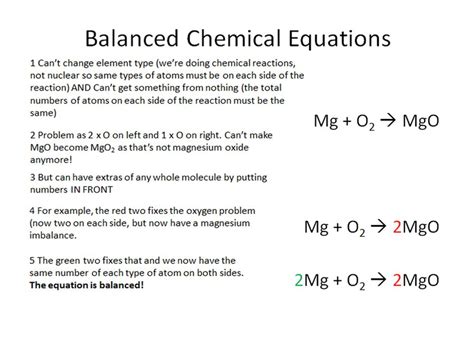
Table of Contents
Why Chemical Equations Must Be Balanced: A Comprehensive Guide
Balancing chemical equations is a fundamental concept in chemistry. It's more than just a classroom exercise; it's a crucial step in understanding chemical reactions and their applications in various fields, from industrial processes to biological systems. This comprehensive guide delves into the reasons why balancing chemical equations is essential, exploring the underlying principles and practical implications.
The Law of Conservation of Mass: The Cornerstone of Balanced Equations
At the heart of balancing chemical equations lies the Law of Conservation of Mass. This fundamental law of nature states that matter cannot be created or destroyed in a chemical reaction. The total mass of the reactants (the starting materials) must be equal to the total mass of the products (the substances formed). If a chemical equation isn't balanced, it violates this fundamental law, rendering it inaccurate and scientifically meaningless.
Visualizing the Law: An Analogy
Imagine baking a cake. You start with specific amounts of flour, sugar, eggs, and other ingredients (reactants). After baking, you have a cake (product). The mass of the cake, along with any leftover ingredients or byproducts (like steam), must equal the total mass of the ingredients you initially used. If you end up with more cake than ingredients, or vice versa, something is fundamentally wrong with your recipe (or your understanding of baking!). This same principle applies to chemical reactions.
The Importance of Balanced Equations: Practical Applications
Balancing equations isn't merely an academic exercise; it has far-reaching implications in various real-world applications:
1. Accurate Stoichiometric Calculations:
Balanced equations provide the stoichiometric ratios between reactants and products. These ratios are crucial for performing accurate calculations in chemistry. For example, in industrial settings, knowing the exact stoichiometric ratios ensures efficient use of reactants, minimizing waste and maximizing product yield. This is vital in processes like fertilizer production, where precise control over reactant ratios is essential for efficiency and cost-effectiveness. Without balanced equations, these calculations would be impossible.
2. Understanding Reaction Mechanisms:
Balancing equations helps chemists understand the mechanism of a reaction. By analyzing the balanced equation, we can infer information about the number of molecules involved, the steps in the reaction process, and the changes in oxidation states. This is crucial in developing new catalysts, improving reaction rates, and designing efficient chemical processes.
3. Predicting Product Yields:
Balanced equations allow us to predict the theoretical yield of a reaction. Knowing the stoichiometric ratios helps in determining how much product can be obtained from a given amount of reactants. This is essential in industrial chemistry for optimizing production processes and managing resource allocation. It's a critical factor in ensuring consistent and reliable product output.
4. Environmental Monitoring and Control:
In environmental science, balanced equations are crucial for understanding and predicting the outcomes of various chemical processes. For example, understanding the stoichiometry of reactions involving pollutants helps in developing effective strategies for environmental remediation and pollution control. This is critical in assessing the impact of industrial emissions and designing effective cleanup strategies.
5. Biochemical Processes:
In biological systems, balanced equations are vital for understanding biochemical pathways. The intricate network of metabolic reactions relies on precise stoichiometric relationships. Understanding these relationships is crucial in fields like drug development, metabolic engineering, and diagnosing metabolic disorders.
The Process of Balancing Chemical Equations: A Step-by-Step Guide
Balancing a chemical equation involves adjusting the coefficients (numbers placed in front of chemical formulas) to ensure that the number of atoms of each element is the same on both sides of the equation. Here's a step-by-step guide:
-
Write the Unbalanced Equation: Start by writing the correct chemical formulas for all reactants and products. This is crucial, as incorrect formulas will lead to an unbalanced equation, regardless of the subsequent steps.
-
Identify the Elements: List all the elements present in the equation.
-
Count the Atoms: Count the number of atoms of each element on both the reactant and product sides of the equation.
-
Balance the Elements: Adjust the coefficients to balance the number of atoms of each element on both sides. Start with elements that appear in only one reactant and one product. Avoid changing the subscripts in the chemical formulas, as this would change the chemical species involved.
-
Verify the Balance: Once you've adjusted the coefficients, double-check to ensure that the number of atoms of each element is equal on both sides of the equation.
-
Check for Simplest Whole-Number Ratios: Make sure the coefficients are in their simplest whole-number ratio. For example, if you end up with coefficients like 2, 4, and 6, reduce them to 1, 2, and 3.
Common Mistakes to Avoid When Balancing Equations
Several common mistakes can lead to unbalanced equations. Understanding these common pitfalls is vital to developing proficiency in balancing equations.
-
Changing Subscripts: Never change the subscripts in a chemical formula. Altering subscripts changes the chemical identity of the substance.
-
Ignoring Polyatomic Ions: When polyatomic ions (like sulfate or phosphate) remain intact throughout the reaction, treat them as a single unit when balancing.
-
Rushing the Process: Balancing equations requires careful attention to detail. Take your time, and carefully review your work after each step.
-
Not Checking Your Work: Always verify your balanced equation by counting the atoms of each element on both sides to confirm equality.
Advanced Techniques for Balancing Complex Equations
For more complex reactions, particularly redox reactions (reactions involving electron transfer), additional techniques are necessary.
-
Oxidation State Method: This method involves assigning oxidation states to each element and tracking the changes in oxidation states during the reaction. The number of electrons gained and lost must balance.
-
Half-Reaction Method: This method involves dividing the overall reaction into two half-reactions, one for oxidation and one for reduction. Each half-reaction is balanced separately, and then the two half-reactions are combined to obtain the overall balanced equation.
Conclusion: The Indispensable Role of Balanced Equations
Balancing chemical equations is not merely a procedural step in chemistry; it's a cornerstone of the discipline. The Law of Conservation of Mass necessitates balanced equations to accurately represent chemical transformations. Their importance extends far beyond the classroom, influencing various scientific and industrial applications, from precise stoichiometric calculations to environmental monitoring and advanced biochemical analyses. Mastering the art of balancing equations is essential for anyone pursuing a deeper understanding of chemistry and its impact on the world. By understanding the principles and applying the techniques discussed in this guide, you can confidently navigate the complexities of chemical reactions and contribute to scientific advancements in various fields.
Latest Posts
Latest Posts
-
Greatest Common Factor Of 10 And 4
Mar 18, 2025
-
How To Know If Something Is A Kite
Mar 18, 2025
-
Greatest Common Factor Of 12 And 18
Mar 18, 2025
-
Classify Whether Each Compound Contains An Ionic Bond
Mar 18, 2025
-
4 1 2 As An Improper Fraction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about Why Chemical Equations Must Be Balanced . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
