Why Are Ionic Compounds Soluble In Water
listenit
Mar 30, 2025 · 6 min read
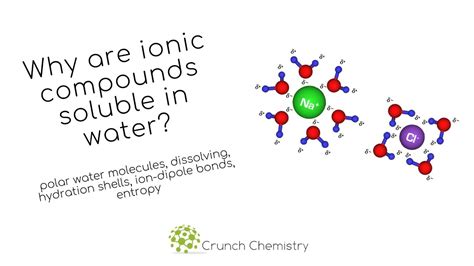
Table of Contents
Why Are Ionic Compounds Soluble in Water? A Deep Dive into Polarity, Hydration, and Solubility
Understanding why ionic compounds dissolve in water is fundamental to chemistry. It's a process governed by the intricate dance between electrostatic forces, molecular polarity, and the unique properties of water itself. This article delves deep into the mechanisms behind this solubility, exploring the concepts of polarity, hydration, and the factors that influence the solubility of ionic compounds in water.
The Polar Nature of Water: The Key to Dissolution
Water (H₂O) is a polar molecule. This means it possesses a slightly positive end and a slightly negative end due to the unequal sharing of electrons between the oxygen and hydrogen atoms. The oxygen atom is more electronegative, attracting electrons more strongly and creating a partial negative charge (δ−). Conversely, the hydrogen atoms carry a partial positive charge (δ+). This uneven distribution of charge creates a dipole moment, making water a powerful solvent for many ionic compounds.
Understanding Electronegativity and Polarity
Electronegativity is the measure of an atom's ability to attract electrons in a chemical bond. A large difference in electronegativity between two atoms results in a polar covalent bond, where the electrons are unequally shared. In contrast, a small difference in electronegativity leads to a nonpolar covalent bond, with relatively equal electron sharing. Water's polarity stems from the significant electronegativity difference between oxygen and hydrogen.
The Role of Hydrogen Bonding
The polarity of water also leads to hydrogen bonding. This is a special type of intermolecular force where a hydrogen atom bonded to a highly electronegative atom (like oxygen) is attracted to another electronegative atom in a different molecule. In water, hydrogen bonds form between the slightly positive hydrogen atoms of one water molecule and the slightly negative oxygen atoms of another. This extensive hydrogen bonding network contributes significantly to water's high boiling point, surface tension, and its exceptional ability to dissolve ionic compounds.
Ionic Compounds: A Crystal Lattice of Charged Particles
Ionic compounds are formed by the electrostatic attraction between positively charged ions (cations) and negatively charged ions (anions). These ions arrange themselves in a highly ordered three-dimensional structure known as a crystal lattice. The strong electrostatic forces holding the lattice together determine many of the physical properties of ionic compounds, including their high melting and boiling points and their often brittle nature.
The Importance of Electrostatic Interactions
The strength of the electrostatic interactions within the crystal lattice depends on several factors:
- Charge magnitude: Higher charges on the ions lead to stronger attractions. For example, the electrostatic attraction between Mg²⁺ and O²⁻ is stronger than that between Na⁺ and Cl⁻.
- Ionic radii: Smaller ions result in stronger attractions because the charges are closer together.
- Lattice energy: This is the energy required to completely separate one mole of a solid ionic compound into its gaseous ions. A high lattice energy indicates strong electrostatic interactions within the crystal lattice.
The Dissolution Process: Hydration and Solvation
The dissolution of an ionic compound in water involves a complex interplay between the forces within the crystal lattice and the interactions between the ions and water molecules. This process is driven by the hydration of ions, a specific type of solvation.
The Hydration of Ions
When an ionic compound is added to water, the polar water molecules surround the individual ions. The slightly negative oxygen atoms of water molecules are attracted to the positive cations, while the slightly positive hydrogen atoms are attracted to the negative anions. This process is called hydration. The water molecules effectively shield the ions from each other, reducing the electrostatic attraction that holds the crystal lattice together.
Breaking the Crystal Lattice: An Energy Balance
The dissolution process involves two competing energy changes:
- Lattice energy: The energy required to break the ionic bonds in the crystal lattice. This is an endothermic process (absorbs energy).
- Hydration energy: The energy released when water molecules surround the ions, forming hydration shells. This is an exothermic process (releases energy).
For an ionic compound to dissolve in water, the hydration energy must be greater than the lattice energy. In other words, the energy released by hydration must exceed the energy required to break the crystal lattice. If the hydration energy is less than the lattice energy, the compound will be insoluble or sparingly soluble in water.
Factors Affecting Solubility of Ionic Compounds in Water
Several factors influence the solubility of ionic compounds in water:
1. Charge Density of Ions
Ions with high charge density (high charge and small size) tend to be more soluble because they interact more strongly with water molecules. This is because a higher charge leads to a stronger attraction to the polar water molecules. Similarly, a smaller size allows for closer interaction with the water molecules.
2. Size of Ions
Larger ions generally have lower charge density and therefore weaker interactions with water molecules. This can lead to lower solubility compared to smaller ions with the same charge.
3. Temperature
The solubility of most ionic compounds increases with increasing temperature. This is because the increased kinetic energy of the water molecules helps to overcome the lattice energy and enhance the hydration process.
4. Pressure
Pressure has a relatively minor effect on the solubility of ionic compounds in water, especially at moderate pressures. However, at very high pressures, the solubility may be slightly affected.
5. Common Ion Effect
The presence of a common ion in the solution reduces the solubility of an ionic compound. This is because the equilibrium between the solid and dissolved ions is shifted towards the solid phase due to Le Chatelier's principle.
Exceptions to the Rule: Insoluble Ionic Compounds
While many ionic compounds are soluble in water, some are insoluble or only sparingly soluble. This occurs when the lattice energy is significantly higher than the hydration energy. Insoluble ionic compounds often precipitate out of solution when their constituent ions are mixed. The solubility rules provide a useful guideline for predicting the solubility of common ionic compounds.
Conclusion: A Balancing Act of Forces
The solubility of ionic compounds in water is a complex process determined by the balance between the energy required to break the crystal lattice and the energy released during ion hydration. The polar nature of water, with its ability to form hydrogen bonds and interact strongly with charged ions, plays a crucial role in this process. Understanding the concepts of polarity, hydration, and the factors that influence solubility allows for a deeper appreciation of the behavior of ionic compounds in aqueous solutions and is fundamental to various aspects of chemistry and related fields. The intricate interplay of electrostatic forces, molecular interactions, and energy changes offers a fascinating glimpse into the molecular world.
Latest Posts
Latest Posts
-
How Much Is 132 Lbs In Kg
Apr 01, 2025
-
How Many 1 8 In A Pound
Apr 01, 2025
-
Identify The Three Quantum Numbers For This Orbital
Apr 01, 2025
-
What Is The Name Of The Positively Charged Subatomic Particle
Apr 01, 2025
-
Why Does Bone Heal Faster Than Cartilage
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about Why Are Ionic Compounds Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
