Why Are Covalent Bonds Soluble In Water
listenit
Mar 23, 2025 · 5 min read
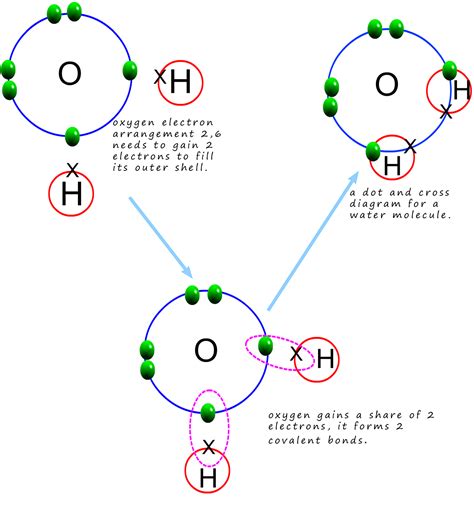
Table of Contents
Why Aren't Covalent Bonds Always Soluble in Water? A Deep Dive into Polarity, Hydrogen Bonding, and Molecular Size
The simple answer to the question "why are covalent bonds soluble in water?" is: they aren't always. While the popular misconception exists that covalent compounds are insoluble, the reality is far more nuanced. Solubility in water is a complex phenomenon governed by the interplay of several factors, primarily the polarity of the covalent molecule and its ability to interact with water's polar nature through hydrogen bonding. Let's delve into the specifics.
Understanding Water's Polar Nature
Water (H₂O) is a polar molecule. This means it has a slightly positive end (the hydrogen atoms) and a slightly negative end (the oxygen atom). This polarity arises due to the difference in electronegativity between oxygen and hydrogen. Oxygen, being more electronegative, attracts the shared electrons more strongly, creating a partial negative charge (δ-) on the oxygen and partial positive charges (δ+) on the hydrogens. This uneven distribution of charge is crucial for understanding its solvent properties.
The Role of Polarity in Covalent Compound Solubility
Covalent compounds dissolve in water when they can interact favorably with water molecules. This favorable interaction primarily involves dipole-dipole interactions and hydrogen bonding.
Dipole-Dipole Interactions
Polar covalent molecules possess a permanent dipole moment, meaning they have a positive and negative end. When these molecules are introduced to water, the positive end of the solute molecule is attracted to the negative end of the water molecule, and vice-versa. These attractions are called dipole-dipole interactions. The stronger these interactions, the greater the solubility.
Hydrogen Bonding: A Special Type of Dipole-Dipole Interaction
Hydrogen bonding is a particularly strong type of dipole-dipole interaction that occurs when a hydrogen atom bonded to a highly electronegative atom (like oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a different molecule. Water molecules are extensively hydrogen-bonded to each other, forming a complex network of interactions. For a covalent molecule to dissolve well in water, it needs to be able to participate in hydrogen bonding with water molecules.
Factors Affecting Solubility of Covalent Compounds
Several factors, beyond simple polarity, determine the solubility of a covalent compound in water:
1. Molecular Polarity: The Primary Determinant
The polarity of the covalent molecule is the most significant factor. Highly polar molecules, like ethanol (CH₃CH₂OH) and glucose (C₆H₁₂O₆), readily dissolve in water because they can form strong hydrogen bonds with water molecules. Nonpolar molecules, like methane (CH₄) and oil, have no significant dipole moment and therefore do not interact favorably with water. They are hydrophobic ("water-fearing") and remain insoluble.
2. Hydrogen Bonding Capability: Enhancing Solubility
The ability to form hydrogen bonds with water significantly enhances solubility. Molecules containing hydroxyl (-OH), amino (-NH₂), and carboxyl (-COOH) groups can participate in hydrogen bonding, making them more likely to be water-soluble. The more hydrogen bonding sites a molecule possesses, the greater its solubility.
3. Molecular Size and Shape: The Role of Steric Hindrance
Molecular size and shape also influence solubility. Larger molecules, even if polar, may have reduced solubility due to steric hindrance. The bulky size can prevent effective interaction with water molecules. Similarly, the shape of the molecule can affect how well it can interact with the water's three-dimensional network of hydrogen bonds. A more compact, spherical shape generally leads to better solubility than a long, chain-like structure.
4. Temperature: The Effect of Kinetic Energy
Temperature affects the solubility of many covalent compounds in water. Increasing the temperature generally increases solubility because it provides more kinetic energy to overcome the intermolecular forces between solute molecules and water molecules. However, this is not always the case, as some compounds exhibit unusual temperature dependence.
5. Presence of Other Functional Groups: The Influence of Non-Polar Regions
The presence of non-polar regions within a molecule can reduce its overall solubility. Even if a molecule possesses polar groups capable of hydrogen bonding, the presence of a large hydrocarbon chain (as seen in fatty acids) can hinder its interaction with water and diminish its solubility.
Examples Illustrating Solubility Variations
Let's examine specific examples to clarify the interplay of these factors:
-
Ethanol (CH₃CH₂OH): Highly soluble in water due to its polar hydroxyl (-OH) group, which participates in strong hydrogen bonding with water.
-
Glucose (C₆H₁₂O₆): Readily dissolves in water due to the presence of multiple hydroxyl (-OH) groups, enabling extensive hydrogen bonding.
-
Methane (CH₄): Insoluble in water because it is a nonpolar molecule and cannot form significant interactions with water molecules.
-
Octanol (CH₃(CH₂)₇OH): Partially soluble in water. While it has a polar hydroxyl group, the long non-polar hydrocarbon chain reduces its overall solubility.
-
Acetic Acid (CH₃COOH): Soluble due to the polar carboxyl (-COOH) group forming hydrogen bonds with water.
-
Benzene (C₆H₆): Insoluble because it is a nonpolar aromatic hydrocarbon.
Misconceptions about Covalent Bonding and Solubility
It's crucial to dispel common misunderstandings:
-
Covalent bonds themselves don't determine solubility: The nature of the covalent bond itself (single, double, triple) is less significant than the overall polarity of the molecule and its ability to interact with water.
-
Not all covalent compounds are insoluble: Many polar covalent compounds readily dissolve in water. The misconception likely stems from encountering many nonpolar organic molecules that are indeed insoluble.
-
Solubility is a spectrum: Solubility isn't a binary property (soluble or insoluble). It's a continuum, ranging from highly soluble to completely insoluble. Many compounds exhibit partial solubility.
Conclusion: A Complex Interplay
The solubility of covalent compounds in water is a complex phenomenon influenced by multiple factors. The most critical factor is the polarity of the molecule and its capacity for hydrogen bonding with water. Molecular size, shape, temperature, and the presence of both polar and nonpolar regions all play a role in determining the extent of solubility. Understanding these factors is essential for predicting and interpreting the behavior of covalent compounds in aqueous solutions. By appreciating the intricacies of molecular interactions, we can more accurately predict and explain the diverse solubility patterns observed in the world around us. This comprehensive understanding is crucial in various fields, from chemistry and biology to medicine and environmental science.
Latest Posts
Latest Posts
-
1 3 Divided By 4 In Fraction
Mar 25, 2025
-
Which Electromagnetic Has The Longest Wavelength
Mar 25, 2025
-
2 1 4 As A Fraction
Mar 25, 2025
-
What Is 4 Percent Of 100
Mar 25, 2025
-
What Percentage Is 7 Of 20
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Why Are Covalent Bonds Soluble In Water . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
