Which Subatomic Particle Determines The Identity Of An Atom
listenit
Mar 21, 2025 · 6 min read
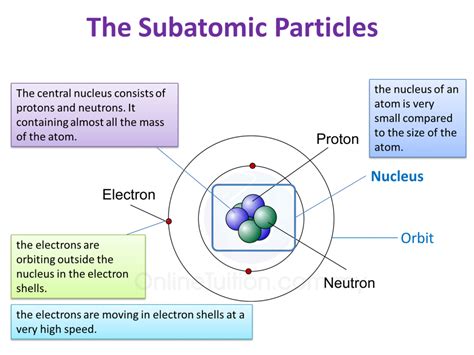
Table of Contents
Which Subatomic Particle Determines the Identity of an Atom?
The atom, the fundamental building block of matter, is a fascinating and complex entity. Understanding its structure is key to comprehending the properties of all substances, from the air we breathe to the stars in the sky. But which subatomic particle truly dictates the identity of an atom? The answer, while seemingly simple, unveils a deeper understanding of the forces and interactions governing the universe. This article delves into the world of subatomic particles, exploring the roles of protons, neutrons, and electrons in defining atomic identity.
The Trio of Subatomic Particles: Protons, Neutrons, and Electrons
Atoms are composed of three primary subatomic particles:
- Protons: Positively charged particles residing within the atom's nucleus.
- Neutrons: Neutral particles (no charge) also found in the nucleus.
- Electrons: Negatively charged particles orbiting the nucleus in shells or energy levels.
While all three contribute to the atom's overall properties, one stands out as the primary determinant of its identity: the proton.
The Proton: The Defining Particle of Atomic Identity
The number of protons in an atom's nucleus defines its atomic number, a fundamental property that uniquely identifies each element on the periodic table. This number is so crucial that it's essentially the atom's "identity card."
Atomic Number: The Key to Element Identification
The atomic number dictates:
- Element Identity: Each element possesses a unique atomic number. For example, hydrogen (H) has an atomic number of 1 (one proton), helium (He) has an atomic number of 2 (two protons), and so on. No two elements share the same atomic number.
- Chemical Properties: The number of protons directly influences the electron configuration, determining how an atom interacts with other atoms—its chemical behavior. The arrangement of electrons in shells dictates bonding capabilities, reactivity, and overall chemical properties.
- Position on the Periodic Table: Elements are arranged on the periodic table in ascending order of their atomic number, reflecting their fundamental properties and relationships.
Isotopes: Variations on a Theme
While the proton number defines the element, the number of neutrons can vary. Atoms of the same element with different numbers of neutrons are called isotopes. Isotopes have the same atomic number (same number of protons) but different mass numbers (total number of protons and neutrons).
For example, carbon-12 (¹²C) has six protons and six neutrons, while carbon-14 (¹⁴C) has six protons and eight neutrons. Both are carbon atoms because they have six protons, but they have different masses and slightly different properties. Isotopes can be stable or unstable (radioactive).
The Role of Neutrons and Electrons
While protons define the element's identity, neutrons and electrons also play crucial roles:
Neutrons: Contributing to Mass and Stability
Neutrons contribute significantly to the atom's mass but not to its charge. Their primary function is to stabilize the nucleus. The strong nuclear force, one of the fundamental forces in nature, holds protons and neutrons together within the nucleus. Without enough neutrons, the electrostatic repulsion between positively charged protons would overcome the strong force, causing the nucleus to become unstable and decay.
The ratio of protons to neutrons influences nuclear stability. For lighter elements, a roughly equal number of protons and neutrons is ideal for stability. However, as the atomic number increases, a greater proportion of neutrons is needed to maintain stability.
Electrons: Dictating Chemical Behavior
Electrons, though much less massive than protons and neutrons, are crucial for determining the atom's chemical properties. The arrangement of electrons in shells or energy levels determines how the atom will interact with other atoms. Electrons participate in chemical bonding, forming molecules and compounds. The outermost shell electrons, called valence electrons, are particularly important in chemical reactions.
The number of electrons in a neutral atom is equal to the number of protons, maintaining overall charge neutrality. However, atoms can gain or lose electrons, forming ions (charged particles). This ability to gain or lose electrons is crucial for chemical bonding and the formation of ionic compounds.
Deeper Dive into Atomic Structure and Quantum Mechanics
To fully appreciate the role of protons in determining atomic identity, it's helpful to delve into a more nuanced understanding of atomic structure, incorporating principles of quantum mechanics:
Quantum Mechanical Model of the Atom
The classical model of the atom, with electrons orbiting the nucleus like planets around the sun, is an oversimplification. The quantum mechanical model provides a more accurate description, introducing concepts like:
- Orbitals: Regions of space where there is a high probability of finding an electron. These orbitals are characterized by specific energy levels and shapes.
- Quantum Numbers: A set of numbers describing the properties of an electron within an orbital, including energy level, orbital shape, and orientation in space.
- Electron Configuration: The arrangement of electrons in the various energy levels and orbitals of an atom. This configuration dictates the atom's chemical behavior.
The proton's role in this quantum mechanical model remains central. The number of protons dictates the number of electrons in a neutral atom, which in turn determines the electron configuration and, consequently, the atom's chemical properties.
The Strong Nuclear Force and Nuclear Stability
The stability of the atomic nucleus, and therefore the atom itself, is governed by the strong nuclear force. This force is responsible for binding protons and neutrons together, overcoming the electrostatic repulsion between protons. The balance between the strong nuclear force and electrostatic repulsion determines whether a nucleus is stable or radioactive. The number of neutrons, therefore, plays a significant role in nuclear stability, influencing the likelihood of radioactive decay.
Beyond Protons: Nuclear Isotopy and Radioactive Decay
Isotopes, atoms of the same element with varying neutron numbers, highlight the complexity of atomic structure and its influence on properties. While the number of protons defines the element, isotopes exhibit differences in mass, stability, and radioactive behavior. Understanding isotopic variation is crucial in fields like nuclear medicine, radioactive dating, and nuclear physics.
Conclusion: The Proton's Reign Supreme
In summary, the proton, with its unique positive charge and its location within the atom's nucleus, is the primary subatomic particle that determines the identity of an atom. Its number, the atomic number, dictates the element's placement on the periodic table, its chemical behavior, and its fundamental properties. While neutrons contribute to mass and nuclear stability, and electrons determine the atom's chemical reactivity, the proton remains the ultimate identifier of atomic identity. This fundamental understanding is essential for comprehending the vast array of chemical and physical properties exhibited by the elements and the world around us, opening doors to further exploration in the fascinating realm of atomic physics and chemistry.
Latest Posts
Latest Posts
-
What Is The Molar Mass Of Calcium Chloride
Mar 28, 2025
-
What Is 3 10 In A Decimal
Mar 28, 2025
-
Whats The Sum Of 2 5 And 2 4
Mar 28, 2025
-
Lewis Dot Structure For Magnesium Chloride
Mar 28, 2025
-
Is Na A Solid Liquid Or Gas
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about Which Subatomic Particle Determines The Identity Of An Atom . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
