Which State Of Matter Takes The Shape Of Its Container
listenit
Apr 03, 2025 · 6 min read
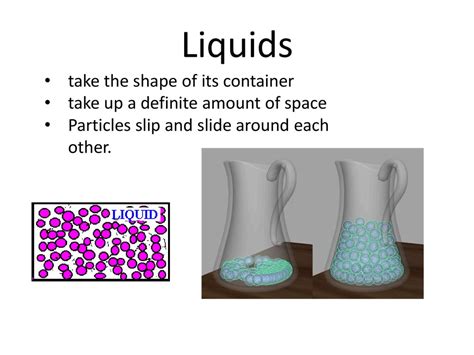
Table of Contents
- Which State Of Matter Takes The Shape Of Its Container
- Table of Contents
- Which State of Matter Takes the Shape of Its Container?
- Understanding the States of Matter
- Solids
- Liquids
- Gases
- Liquids: Conformity to Container Shape
- Gases: Complete Adaptability
- Other States of Matter: Plasmas and Bose-Einstein Condensates
- Exceptions and Considerations
- Conclusion
- Latest Posts
- Latest Posts
- Related Post
Which State of Matter Takes the Shape of Its Container?
The question of which state of matter conforms to the shape of its container is a fundamental concept in chemistry and physics. While seemingly simple, a deeper understanding reveals nuances and exceptions that make it a fascinating topic to explore. The short answer is liquids and gases. However, a thorough examination requires delving into the properties of each state of matter and considering the conditions under which they exist.
Understanding the States of Matter
Before diving into the specifics, let's briefly review the three primary states of matter: solid, liquid, and gas. Each state is characterized by the arrangement and movement of its constituent particles (atoms, molecules, or ions).
Solids
Solids possess a fixed shape and volume. Their particles are closely packed together in a highly ordered arrangement, held in place by strong intermolecular forces. This rigid structure prevents solids from easily changing their shape or volume. Think of a block of ice – its shape and volume remain constant unless subjected to external forces like melting or breaking.
Liquids
Liquids have a fixed volume but take the shape of their container. Their particles are still relatively close together but have enough kinetic energy to move past each other, allowing them to flow and adjust to the container's form. While the volume remains consistent, the liquid will mold itself to fit any container it’s placed into. Imagine pouring water into a glass – the water adopts the shape of the glass, filling the bottom first and rising to match the glass's contours.
Gases
Gases have neither a fixed shape nor a fixed volume. Their particles are widely dispersed and move rapidly and independently of one another, possessing high kinetic energy. They easily compress and expand to fill the available space within their container, always adopting both the shape and volume of their surroundings. Consider a balloon filled with air – the air expands to fill the balloon’s entire space, taking on its shape and volume.
Liquids: Conformity to Container Shape
The ability of a liquid to conform to its container's shape is a direct result of its unique properties. Several factors contribute to this behavior:
-
Intermolecular Forces: While weaker than in solids, intermolecular forces in liquids still hold the particles together, preventing complete dispersion. These forces are responsible for surface tension, the phenomenon that causes liquids to form droplets and minimize their surface area. Despite these forces, the particles possess sufficient kinetic energy to move and slide past one another, allowing them to rearrange and conform to the shape of the container.
-
Fluid Nature: Liquids are considered fluids, meaning they can flow and change their shape under the influence of applied forces (like gravity). This property allows liquids to adapt to the geometry of any vessel they are poured into. The liquid's particles constantly interact, shifting and adjusting to minimize their overall energy, effectively filling the container.
-
Density and Viscosity: The density and viscosity of a liquid influence the speed at which it adapts to the container's shape. Less dense liquids flow more readily, conforming to the container's shape quickly. Highly viscous liquids, such as honey, flow much slower and take longer to conform to the container. However, given sufficient time, even highly viscous liquids will eventually conform to the shape of the vessel.
Gases: Complete Adaptability
Gases exhibit an even greater degree of adaptability compared to liquids. Their particles' high kinetic energy allows them to completely fill any container, regardless of its shape or volume. This is due to several factors:
-
Weak Intermolecular Forces: The intermolecular forces between gas particles are extremely weak, almost negligible. This allows particles to move freely and independently, unaffected by their neighbors.
-
Compressibility and Expansibility: Gases are highly compressible and expansible. Their volume readily changes with pressure and temperature. If the pressure increases, the particles are forced closer together. Conversely, a decrease in pressure allows the particles to spread out and occupy a larger volume. This is why gases readily adapt to the shape and volume of their container.
-
Random Motion: Gas particles exhibit random motion, moving in straight lines until they collide with each other or the container walls. These collisions cause the particles to change direction, further contributing to their ability to uniformly fill the entire space available.
Other States of Matter: Plasmas and Bose-Einstein Condensates
While solid, liquid, and gas are the most commonly encountered states of matter, there are others, including plasmas and Bose-Einstein condensates. Their behavior with respect to container shape is different:
-
Plasmas: Plasmas are ionized gases, meaning they consist of free-moving electrons and ions. Like gases, plasmas tend to fill the entire volume of their container. However, the presence of charged particles introduces additional complexity, including magnetic field effects that can influence their behavior and distribution within the container.
-
Bose-Einstein Condensates: At extremely low temperatures, some substances can form a Bose-Einstein condensate, a state of matter where a large fraction of the particles occupy the lowest quantum state. In this state, the particles behave as a single quantum entity, losing their individual identity. The behavior of a Bose-Einstein condensate concerning its container shape is dependent on the specifics of the trapping potential used to create and confine the condensate. Essentially, it will conform to the boundaries of the trapping field.
Exceptions and Considerations
While the general rule is that liquids and gases conform to the shape of their container, some exceptions and nuances exist:
-
Surface Tension: Surface tension can cause a meniscus, the curved surface of a liquid in a container. This curvature is a result of the intermolecular forces within the liquid and its interaction with the container's surface. While the liquid conforms to the overall shape, the surface isn't perfectly flat.
-
Capillary Action: Capillary action is the phenomenon where a liquid spontaneously rises or falls in a narrow tube. This effect is caused by the interplay between adhesive forces (between the liquid and the tube) and cohesive forces (within the liquid). This means that the shape of the liquid inside a very narrow tube might not precisely mirror the tube's shape at the microscopic level.
-
Highly Viscous Liquids: Extremely viscous liquids may take a considerable amount of time to fully conform to their container's shape. While they will eventually adjust, the process may be slow enough to appear as if they are not fully adapting.
Conclusion
In conclusion, liquids and gases are the states of matter that readily take the shape of their container. This is due to their differing degrees of intermolecular forces, particle movement, and density. Understanding this fundamental property requires considering various factors such as intermolecular forces, kinetic energy, viscosity, and pressure. While exceptions and nuances exist, the primary characteristic of liquids and gases is their adaptability to the shape of any vessel they occupy. The behavior of other states of matter, such as plasmas and Bose-Einstein condensates, further enriches our understanding of matter's diverse characteristics and their interaction with their surroundings. This fundamental concept underscores the dynamic and fascinating world of states of matter and their properties.
Latest Posts
Latest Posts
-
What Does An Exclamation Mark Mean In Math
Apr 05, 2025
-
How Many Suns In The Universe
Apr 05, 2025
-
How To Calculate The Net Electric Field
Apr 05, 2025
-
How Many Electrons Can Be Held In The Third Orbital
Apr 05, 2025
-
What Is The Importance Of Having The Anatomical Position
Apr 05, 2025
Related Post
Thank you for visiting our website which covers about Which State Of Matter Takes The Shape Of Its Container . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
