Which Is The Most Abundant Gas In The Atmosphere
listenit
Mar 19, 2025 · 5 min read
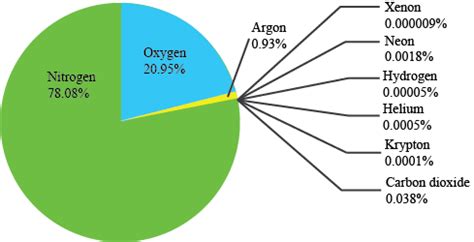
Table of Contents
Which is the Most Abundant Gas in the Atmosphere?
The Earth's atmosphere is a complex mixture of gases, each playing a vital role in shaping our planet's climate, weather patterns, and the very existence of life as we know it. While many gases contribute to the atmospheric composition, one stands out as the most abundant: nitrogen. Understanding the prevalence of nitrogen and the roles of other atmospheric gases is crucial for appreciating the delicate balance of our planet's environment. This article will delve into the composition of the atmosphere, focusing on nitrogen's dominance and the significant contributions of other gases.
The Dominant Player: Nitrogen (N₂)
Nitrogen, represented chemically as N₂, makes up approximately 78% of Earth's atmosphere by volume. This incredibly high percentage underscores its critical role in atmospheric processes and the global ecosystem. However, despite its abundance, elemental nitrogen in its gaseous form (N₂) is largely inert. This means it doesn't readily react with other substances under normal conditions. This characteristic is both a blessing and a challenge.
Why is Nitrogen Inert?
The strong triple covalent bond between the two nitrogen atoms in the N₂ molecule requires a significant amount of energy to break. This makes it difficult for nitrogen to participate in many chemical reactions. While this inertness protects it from rapidly depleting, it also limits its direct availability for biological processes.
The Nitrogen Cycle: From Inert Gas to Life's Building Block
The inertness of atmospheric nitrogen is circumvented by a crucial biogeochemical cycle known as the nitrogen cycle. This cycle involves a series of biological and chemical processes that convert atmospheric nitrogen into usable forms for living organisms. These processes include:
- Nitrogen fixation: Specialized bacteria, found in soil and the roots of certain plants (legumes), convert atmospheric N₂ into ammonia (NH₃) and other nitrogen-containing compounds. This process is essential for making nitrogen available to plants and the wider food chain.
- Nitrification: Other bacteria convert ammonia into nitrites (NO₂⁻) and then nitrates (NO₃⁻), which are readily absorbed by plants.
- Assimilation: Plants absorb nitrates and incorporate them into their tissues, forming essential organic molecules like amino acids and proteins. Animals obtain nitrogen by consuming plants or other animals.
- Ammonification: When organisms die, decomposers break down organic matter, releasing nitrogen back into the soil as ammonia.
- Denitrification: Certain bacteria convert nitrates back into nitrogen gas (N₂), which is released into the atmosphere.
This intricate cycle ensures a continuous supply of usable nitrogen for all living organisms while maintaining a relatively stable atmospheric concentration of N₂.
Oxygen (O₂): The Second Most Abundant Gas
After nitrogen, oxygen (O₂) is the second most abundant gas in the atmosphere, accounting for approximately 21% of its volume. Unlike nitrogen, oxygen is highly reactive, playing a crucial role in many chemical processes.
Oxygen's Vital Role in Respiration and Combustion
Oxygen is essential for aerobic respiration, the process by which most living organisms generate energy from food. This process involves the oxidation of organic molecules, with oxygen acting as the final electron acceptor. Oxygen is also vital for combustion, a rapid chemical reaction that releases energy in the form of heat and light.
The Oxygen Cycle: A Dynamic Balance
The oxygen cycle is closely intertwined with the carbon cycle and other biogeochemical cycles. Photosynthesis, the process by which plants convert light energy into chemical energy, is the primary source of atmospheric oxygen. During photosynthesis, plants absorb carbon dioxide (CO₂) and release oxygen as a byproduct. Respiration, decomposition, and combustion consume oxygen and release carbon dioxide, balancing the cycle.
Argon (Ar): A Noble Gas of Significance
Argon (Ar) is the third most abundant gas in the atmosphere, constituting about 0.93% of its volume. Argon is a noble gas, meaning it is chemically inert and rarely reacts with other substances. Its presence in the atmosphere is primarily due to the radioactive decay of potassium-40 in the Earth's crust.
Other Atmospheric Gases and Their Importance
While nitrogen, oxygen, and argon dominate the atmospheric composition, several other gases play significant roles:
Carbon Dioxide (CO₂): A Greenhouse Gas with Growing Concern
Carbon dioxide (CO₂), though present in relatively small amounts (approximately 0.04%), is a crucial greenhouse gas. It absorbs infrared radiation, trapping heat within the atmosphere and contributing to the Earth's greenhouse effect. Human activities, particularly the burning of fossil fuels, have significantly increased atmospheric CO₂ concentrations, leading to concerns about global warming and climate change.
Water Vapor (H₂O): Variable but Crucial
Water vapor (H₂O) is a highly variable component of the atmosphere, ranging from near zero to several percent by volume, depending on location and weather conditions. It plays a crucial role in the water cycle, weather patterns, and the Earth's energy balance, acting as another significant greenhouse gas.
Ozone (O₃): A Protective Shield
Ozone (O₃) is a highly reactive molecule present in trace amounts in the atmosphere. In the stratosphere, the ozone layer absorbs harmful ultraviolet (UV) radiation from the sun, protecting life on Earth. However, human-made chemicals, such as chlorofluorocarbons (CFCs), have damaged the ozone layer, leading to international efforts to regulate their use.
Other Trace Gases
Several other trace gases, including methane (CH₄), nitrous oxide (N₂O), and various other pollutants, are present in the atmosphere in smaller concentrations. Even though they represent only a tiny fraction of the atmosphere's total volume, these gases can have significant impacts on climate and air quality.
Conclusion: The Atmospheric Symphony
The Earth's atmosphere is a dynamic and interconnected system. While nitrogen holds the title of the most abundant gas, its role is intrinsically linked to the activities of oxygen, carbon dioxide, water vapor, and other gases. Understanding the composition and interactions of these gases is vital for comprehending climate change, environmental protection, and the preservation of life on Earth. The atmosphere’s intricate balance underscores the interconnectedness of Earth’s systems and the critical importance of maintaining a healthy planet. Further research and global cooperation are crucial to mitigate the negative impacts of human activities on atmospheric composition and ensure a sustainable future. The dominance of nitrogen, while significant, only tells part of the story—a story that involves the complex interplay of numerous gases, each with a unique and vital role to play.
Latest Posts
Latest Posts
-
What Is The Greatest Common Divisor Of 24 And 32
Mar 19, 2025
-
A Certain Substance Has A Heat Of Vaporization Of
Mar 19, 2025
-
Equation For Newtons Law Of Cooling
Mar 19, 2025
-
What Is The Monomer That Makes Up Nucleic Acids
Mar 19, 2025
-
A Flag Pole Is Supported By Two Wires
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about Which Is The Most Abundant Gas In The Atmosphere . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
