Which Gas Is Most Abundant In Earth's Atmosphere
listenit
Mar 23, 2025 · 6 min read
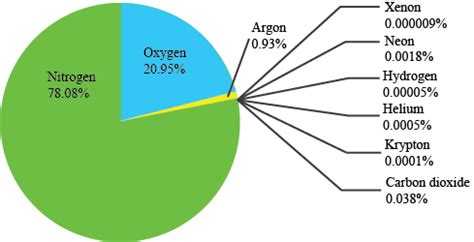
Table of Contents
Which Gas is Most Abundant in Earth's Atmosphere? A Deep Dive into Atmospheric Composition
Earth's atmosphere, the gaseous envelope surrounding our planet, is a complex mixture of various gases. Understanding its composition is crucial for comprehending weather patterns, climate change, and the very existence of life on Earth. While many gases contribute to the atmospheric mix, one stands out as the most abundant: nitrogen (N₂). This article delves deep into the composition of Earth's atmosphere, focusing on the dominance of nitrogen, its role, and the significance of other key atmospheric components.
The Predominance of Nitrogen: A 78% Share
Nitrogen gas (N₂) constitutes approximately 78% of Earth's atmosphere by volume. This incredibly high percentage highlights its fundamental role in shaping our planet's environment. Unlike oxygen, which is highly reactive and plays a vital role in biological processes, nitrogen's relative inertness contributes to its abundance. Its strong triple bond makes it difficult to react with other elements under normal atmospheric conditions.
The Inert Nature of Nitrogen: A Blessing and a Curse
While nitrogen's inertness is responsible for its abundance, it also presents a challenge. Most organisms cannot directly utilize atmospheric nitrogen. This inert nature necessitates the involvement of specialized microorganisms called nitrogen-fixing bacteria. These bacteria possess the enzymes necessary to break the strong triple bond in nitrogen gas and convert it into usable forms, primarily ammonia (NH₃), which plants can then assimilate. This process, known as nitrogen fixation, is a cornerstone of the nitrogen cycle, essential for life on Earth.
The Nitrogen Cycle: A Continuous Process
The nitrogen cycle involves a complex interplay of biological, chemical, and physical processes. Nitrogen fixation is the starting point, converting atmospheric nitrogen into biologically available forms. These forms are then incorporated into plants, animals, and other organisms. Once incorporated, nitrogen is released back into the atmosphere through processes such as decomposition, denitrification (conversion of nitrates back to nitrogen gas), and ammonia volatilization. The continuous cycling of nitrogen maintains its presence in the atmosphere while supplying organisms with a critical nutrient.
Oxygen: The Second Most Abundant Gas and Essential for Life
Following nitrogen, oxygen (O₂) holds the second position, accounting for approximately 21% of Earth's atmosphere. Unlike nitrogen, oxygen is highly reactive and crucial for respiration in most organisms. It's essential for the energy production processes within cells and supports numerous other biological functions. The presence of oxygen in the atmosphere is largely attributed to photosynthesis by plants and other photosynthetic organisms, which release oxygen as a byproduct.
The Role of Photosynthesis: Oxygen Production and Carbon Dioxide Consumption
Photosynthesis is the cornerstone of Earth's oxygen production. Through this process, plants and other organisms use sunlight, water, and carbon dioxide (CO₂) to produce glucose (a sugar) and oxygen. This process not only provides oxygen for respiration but also plays a vital role in regulating atmospheric carbon dioxide levels. The balance between photosynthesis and respiration is crucial for maintaining the delicate equilibrium of atmospheric gases.
The Importance of Oxygen in Biological Processes
Oxygen's reactivity is central to its biological significance. It serves as the final electron acceptor in the process of cellular respiration, generating the energy needed for various life processes. Its involvement in combustion (burning) is also essential for many industrial and natural processes. However, oxygen's reactivity also contributes to its role in oxidative stress, which can damage cellular components. Organisms have developed mechanisms to protect themselves from the harmful effects of excessive oxygen.
Argon: The Third Most Abundant Gas and a Noble Gas
Argon (Ar) makes up approximately 0.93% of Earth's atmosphere. It's a noble gas, meaning it's chemically inert and rarely reacts with other elements. Argon is a byproduct of radioactive decay of potassium-40 in the Earth's crust. Its inertness prevents it from participating in many chemical processes in the atmosphere, thereby maintaining its consistent presence.
Noble Gases: Chemically Unreactive and Abundant in Trace Amounts
In addition to argon, other noble gases—helium (He), neon (Ne), krypton (Kr), xenon (Xe), and radon (Rn)—are present in trace amounts in the atmosphere. These gases are all chemically inert and play minimal roles in atmospheric processes. Their abundance in the atmosphere is typically very low compared to nitrogen, oxygen, and argon.
Carbon Dioxide: A Trace Gas with a Significant Impact
Carbon dioxide (CO₂), while present in a relatively small concentration of about 0.04%, is a critical component of Earth's atmosphere. It plays a crucial role in the Earth's climate system through the greenhouse effect. CO₂ absorbs and re-emits infrared radiation, trapping heat within the atmosphere. Increased levels of atmospheric CO₂ due to human activities are a major driver of global warming and climate change.
The Greenhouse Effect: A Natural Process with Anthropogenic Influence
The greenhouse effect is a natural process that helps regulate Earth's temperature. Gases like CO₂, methane (CH₄), nitrous oxide (N₂O), and water vapor trap heat and prevent it from escaping into space. However, human activities, especially the burning of fossil fuels, have significantly increased atmospheric CO₂ levels, enhancing the greenhouse effect and leading to global warming.
The Importance of Understanding Carbon Dioxide Levels
Monitoring and understanding atmospheric carbon dioxide levels are critical for predicting and mitigating the effects of climate change. Scientists use various techniques, such as ice core analysis and direct atmospheric measurements, to track changes in CO₂ concentrations over time. This information is crucial for informing policies and actions aimed at reducing greenhouse gas emissions and mitigating climate change.
Other Atmospheric Constituents: Water Vapor and Trace Gases
In addition to the major components discussed above, several other gases are present in smaller amounts. Water vapor (H₂O) is a highly variable component, ranging from near zero to around 4% depending on location and weather conditions. Water vapor is crucial for weather patterns, cloud formation, and the hydrological cycle.
Other trace gases, such as ozone (O₃), methane (CH₄), nitrous oxide (N₂O), and various aerosols, play important roles in atmospheric chemistry and climate. Ozone, for example, is vital in the stratosphere where it protects us from harmful ultraviolet radiation, but in the troposphere (lower atmosphere) it is a pollutant contributing to smog. Methane and nitrous oxide are potent greenhouse gases, contributing to climate change.
Conclusion: The Dynamic Nature of Earth's Atmosphere
Earth's atmosphere is a dynamic and complex system, with nitrogen as the most abundant gas. While nitrogen's inertness contributes to its abundance, the other gases, especially oxygen and carbon dioxide, play crucial roles in sustaining life and influencing the planet's climate. Understanding the composition and interactions within the atmosphere is essential for addressing environmental challenges, such as climate change, and for maintaining a healthy planet. Continuous monitoring and research are crucial to deepen our understanding of the intricate processes influencing the balance and future of Earth's atmosphere. The interplay between these gases, driven by both natural processes and human activities, continuously shapes our planet's environment and underscores the significance of environmental stewardship for future generations. The study of Earth's atmosphere is a continuing quest to unravel its mysteries and predict future trends impacting life on Earth.
Latest Posts
Latest Posts
-
What Is 25 As A Decimal
Mar 25, 2025
-
What Is The Decimal Of 4 6
Mar 25, 2025
-
How To Write Electron Configuration For Ions
Mar 25, 2025
-
How Many Feet Are In 0 4 Miles
Mar 25, 2025
-
What Do Letters Dna Stand For
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Gas Is Most Abundant In Earth's Atmosphere . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
