Which Color Of Light Has The Highest Energy
listenit
Mar 23, 2025 · 5 min read
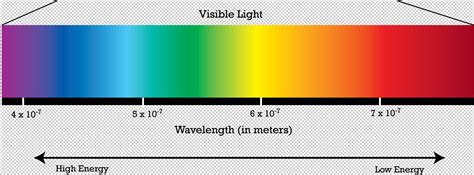
Table of Contents
Which Color of Light Has the Highest Energy? Understanding the Electromagnetic Spectrum
The question of which color of light possesses the highest energy is fundamental to understanding the nature of light and its interaction with matter. While seemingly simple, the answer delves into the fascinating world of the electromagnetic spectrum, revealing a relationship between color, wavelength, frequency, and energy that governs much of our physical reality. This comprehensive article will explore this relationship in detail, explaining the underlying physics and providing practical applications of this knowledge.
Understanding the Electromagnetic Spectrum
Light, as we perceive it, is just a small portion of a much broader spectrum of electromagnetic radiation. This spectrum encompasses a vast range of wavelengths, from incredibly long radio waves to incredibly short gamma rays. Visible light, the range our eyes can detect, sits neatly in the middle of this spectrum. Within this visible light spectrum, we perceive different wavelengths as different colors.
Wavelength, Frequency, and Energy: The Trifecta
The key to understanding the energy of light lies in its relationship with wavelength and frequency. These three properties are inextricably linked:
-
Wavelength (λ): This is the distance between two consecutive crests (or troughs) of a light wave. It's typically measured in nanometers (nm). Longer wavelengths correspond to lower energy.
-
Frequency (ν): This refers to the number of complete wave cycles that pass a given point per second. It's measured in Hertz (Hz). Higher frequencies correspond to higher energy.
-
Energy (E): The energy of a photon of light is directly proportional to its frequency. This relationship is described by Planck's equation: E = hν, where 'h' is Planck's constant (a fundamental constant in quantum mechanics).
The Relationship Between Color and Energy
As we move across the visible light spectrum, from red to violet, the wavelength decreases, and the frequency increases. This directly translates to a change in energy. Violet light has the shortest wavelength and the highest frequency, therefore, it possesses the highest energy within the visible light spectrum.
The Visible Light Spectrum: A Detailed Look
Let's break down the visible light spectrum and its energy levels:
- Red: Longest wavelength, lowest frequency, lowest energy.
- Orange: Slightly shorter wavelength, higher frequency, higher energy than red.
- Yellow: Shorter wavelength, higher frequency, higher energy than orange.
- Green: Shorter wavelength, higher frequency, higher energy than yellow.
- Blue: Shorter wavelength, higher frequency, higher energy than green.
- Indigo: Shorter wavelength, higher frequency, higher energy than blue.
- Violet: Shortest wavelength, highest frequency, highest energy.
Beyond Visible Light: Higher Energy Radiations
While violet light holds the highest energy within the visible spectrum, it's crucial to remember that the electromagnetic spectrum extends far beyond what our eyes can see. Beyond violet lie ultraviolet (UV) radiation, X-rays, and gamma rays. These forms of radiation have significantly shorter wavelengths, higher frequencies, and therefore, much higher energies than visible light.
Ultraviolet (UV) Radiation: Sunburns and More
UV radiation, invisible to the human eye, is responsible for sunburns and plays a crucial role in vitamin D synthesis. Its higher energy compared to visible light is what makes it capable of damaging DNA and causing skin cancer. UV radiation is further categorized into UVA, UVB, and UVC, with UVC possessing the highest energy within the UV range.
X-rays: Penetrating Power
X-rays have even shorter wavelengths and higher frequencies than UV radiation. Their high energy allows them to penetrate soft tissues, making them invaluable in medical imaging. The higher the energy of the X-ray, the greater its penetration power.
Gamma Rays: The Most Energetic Radiation
Gamma rays are the most energetic form of electromagnetic radiation. They have incredibly short wavelengths and incredibly high frequencies. Their high energy makes them extremely dangerous, capable of causing significant damage to living tissue. Gamma rays are emitted from radioactive materials and are also produced during nuclear reactions.
Practical Applications of Light Energy
The energy of light, across the entire electromagnetic spectrum, has a wide range of practical applications:
Photovoltaic Cells (Solar Cells): Harnessing Solar Energy
Solar cells convert the energy of sunlight (primarily visible light) into electricity. The efficiency of a solar cell is influenced by its ability to absorb photons of different energies. More efficient solar cells are designed to capture a broader range of wavelengths, including those in the infrared and ultraviolet regions.
Photography and Imaging: Capturing Light
Cameras capture images by converting the energy of light into electrical signals. Different wavelengths of light produce different colors in the image. The sensitivity of a camera's sensor to different wavelengths impacts the quality and accuracy of the resulting image.
Medical Treatments: Lasers and Photodynamic Therapy
Lasers utilize monochromatic (single-wavelength) light of specific energy to perform various medical procedures, including surgery, skin treatments, and cancer therapy. Photodynamic therapy uses light energy to activate drugs to kill cancer cells. The choice of wavelength is crucial in determining the depth of penetration and effectiveness of the treatment.
Spectroscopy: Analyzing Light to Identify Substances
Spectroscopy analyzes the interaction of light with matter. By analyzing the absorption and emission of light at different wavelengths, scientists can identify the composition of various substances. This technique is widely used in chemistry, astronomy, and environmental science.
Conclusion: A Spectrum of Energy and Applications
The question of which color of light possesses the highest energy leads us on a fascinating journey through the electromagnetic spectrum. While violet light holds the highest energy within the visible range, the spectrum extends far beyond, encompassing much higher energy radiations like UV, X-rays, and gamma rays. Understanding the relationship between wavelength, frequency, and energy is crucial for numerous scientific and technological advancements. The ability to harness and manipulate the energy of light continues to drive innovation across various fields, from renewable energy to medical treatments and beyond. The energy inherent in light is not just a scientific curiosity; it's a powerful force shaping our world and our future.
Latest Posts
Latest Posts
-
How Many 3rds In A Cup
Mar 25, 2025
-
Can Travel Through A Empty Space
Mar 25, 2025
-
How To Graph A Rose Curve
Mar 25, 2025
-
The Basic Unit Of Mass In The Metric System
Mar 25, 2025
-
Lowest Common Multiple Of 5 And 10
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about Which Color Of Light Has The Highest Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
