Which Change Of State Involves A Release Of Energy
listenit
Mar 22, 2025 · 6 min read
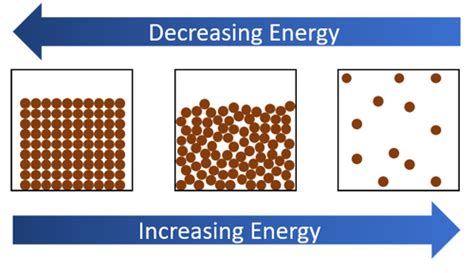
Table of Contents
Which Change of State Involves a Release of Energy? Understanding Exothermic Phase Transitions
The world around us is in constant flux, with matter undergoing continuous transformations. One fundamental aspect of these transformations is the change of state, also known as a phase transition. These changes involve the rearrangement of molecules within a substance, transitioning between solid, liquid, and gaseous phases. Crucially, these transitions are accompanied by energy changes – either absorbing or releasing energy. This article will delve deep into the fascinating world of phase transitions, focusing specifically on those that involve a release of energy, a process known as exothermic changes.
Understanding Phase Transitions and Energy
Before we dive into the specific exothermic changes of state, let's establish a foundational understanding of phase transitions and their relationship to energy. The three primary states of matter – solid, liquid, and gas – are characterized by the degree of molecular organization and the strength of intermolecular forces.
- Solids: Molecules are tightly packed and held together by strong intermolecular forces, resulting in a rigid structure with a fixed shape and volume.
- Liquids: Molecules are closer together than in gases but have more freedom of movement, resulting in a definite volume but an indefinite shape. Intermolecular forces are weaker than in solids.
- Gases: Molecules are widely dispersed with weak intermolecular forces, leading to indefinite shape and volume. They readily expand to fill their containers.
Transitions between these states involve overcoming or establishing these intermolecular forces, which require either the input or release of energy. This energy is typically manifested as heat.
Exothermic Phase Transitions: A Release of Energy
Exothermic processes are those that release energy to their surroundings. In the context of phase transitions, this means the process releases heat into the environment as it occurs. The energy released comes from the formation of stronger intermolecular bonds or a more ordered arrangement of molecules. The opposite, endothermic processes, absorb energy. Let's examine the exothermic phase transitions:
1. Deposition: Gas to Solid
Deposition is the direct transition from the gaseous phase to the solid phase, bypassing the liquid phase. This process is inherently exothermic because the gaseous molecules, which are highly dispersed and have weak intermolecular forces, must lose energy to become organized into a rigid solid structure with strong intermolecular forces. Think of the formation of frost on a cold winter's morning. Water vapor in the air directly transforms into ice crystals on surfaces, releasing heat in the process.
Examples of Deposition:
- Frost formation: Water vapor in the air deposits directly onto cold surfaces as ice crystals.
- Snow formation: Water vapor in clouds directly deposits as snow crystals.
- Formation of thin films: Certain materials can be deposited as thin films in a vacuum deposition process, where the gaseous molecules lose energy and solidify onto a substrate.
2. Condensation: Gas to Liquid
Condensation is the phase transition from the gaseous state to the liquid state. Similar to deposition, condensation is exothermic because the freely moving gas molecules must lose kinetic energy to become more closely packed and interact with stronger intermolecular forces in the liquid phase. The energy released is often perceived as the warmth felt near condensing steam.
Examples of Condensation:
- Dew formation: Water vapor in the air condenses on cooler surfaces as liquid water.
- Cloud formation: Water vapor in the atmosphere condenses to form water droplets or ice crystals in clouds.
- Steam condensation: Water vapor (steam) releases heat as it condenses back into liquid water.
- Fog formation: Condensation of water vapor in the lower atmosphere creates fog.
3. Freezing: Liquid to Solid
Freezing is the phase transition from the liquid state to the solid state. This exothermic process involves the loss of kinetic energy by the liquid molecules, leading to a more ordered, rigid structure with stronger intermolecular forces. As molecules lose energy, they become less mobile and settle into a fixed arrangement, resulting in a solid. The heat released during freezing is responsible for the cooling effect observed.
Examples of Freezing:
- Water freezing into ice: Liquid water releases heat as it freezes into ice at 0°C (32°F).
- Molten metal solidification: Molten metals release significant heat as they solidify into solid metals, a process crucial in metallurgy.
- Freezing of biological materials: The freezing of biological samples involves controlled cooling to minimize damage from ice crystal formation and release of latent heat.
Understanding Latent Heat and Exothermic Changes
The energy released or absorbed during a phase transition is known as latent heat. This heat is "hidden" because it doesn't change the temperature of the substance; instead, it's used to overcome the intermolecular forces involved in the phase change. In exothermic changes of state, the latent heat is released into the surrounding environment. The amount of latent heat released is specific to the substance and the type of phase transition.
Exothermic Phase Transitions in Everyday Life and Nature
Exothermic phase transitions are not just laboratory phenomena; they are integral to many natural processes and everyday occurrences. Let's explore some examples:
In Nature:
- Weather patterns: Condensation and deposition are essential components of weather patterns, influencing cloud formation, precipitation, and temperature changes.
- Formation of geological formations: The solidification of magma into igneous rocks is a massive exothermic process that shapes the Earth's surface.
- Biological processes: Freezing of water in organisms during winter is a vital survival mechanism for certain species.
In Everyday Life:
- Cooling systems: Refrigerators and air conditioners utilize condensation and evaporation (an endothermic process) to create a cooling effect, but the overall system relies on heat released during condensation.
- Food preservation: Freezing food prevents microbial growth by reducing the kinetic energy of microorganisms and slowing down their metabolic activities. The heat released during freezing helps to cool the surrounding environment.
- Industrial processes: Many industrial processes utilize exothermic phase transitions for various purposes, such as the solidification of plastics and the production of certain materials.
Factors Affecting Exothermic Phase Transitions
Several factors can influence the rate and extent of exothermic phase transitions:
- Temperature: Lower temperatures generally favor exothermic phase transitions.
- Pressure: Increased pressure generally favors phase transitions towards denser states (solid or liquid).
- Presence of impurities: Impurities can alter the melting and freezing points of substances, potentially affecting the rate of phase transitions.
- Surface area: A larger surface area generally leads to faster phase transitions.
Conclusion: The Importance of Exothermic Phase Transitions
Exothermic phase transitions are fundamental processes that shape our world, from the formation of weather patterns to the manufacturing of everyday materials. Understanding these processes is crucial for advancements in various fields, including meteorology, materials science, and engineering. The release of energy during these transitions is not simply a byproduct; it's a driving force behind many natural and technological phenomena, highlighting the intricate interplay between energy and matter in the physical world. By understanding the principles governing exothermic phase transitions, we can gain a deeper appreciation for the dynamic and ever-changing nature of our environment. Furthermore, understanding these processes helps us harness and control them for various applications, ranging from improving food preservation to developing more efficient industrial processes. The ongoing research and exploration of these processes continue to unveil their significance in various fields and contribute to new innovations.
Latest Posts
Latest Posts
-
How Many Neutrons Does K Have
Mar 23, 2025
-
How Many Pounds In 1 Pint
Mar 23, 2025
-
How Many Electrons Are In O2
Mar 23, 2025
-
What Is 60 In Fraction Form
Mar 23, 2025
-
63 Is 90 Of What Number
Mar 23, 2025
Related Post
Thank you for visiting our website which covers about Which Change Of State Involves A Release Of Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
