Where On The Periodic Table Are Metals Found
listenit
Apr 01, 2025 · 6 min read
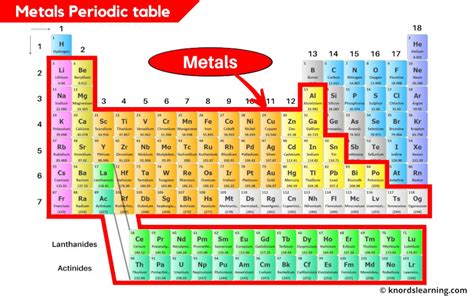
Table of Contents
Where on the Periodic Table are Metals Found? A Comprehensive Guide
The periodic table, a cornerstone of chemistry, organizes elements based on their atomic number and recurring chemical properties. One of the most fundamental classifications of elements is the division into metals, nonmetals, and metalloids. Understanding where metals are located on the periodic table is crucial for grasping their properties and predicting their behavior in chemical reactions. This comprehensive guide delves into the location of metals on the periodic table, exploring their characteristics and offering insights into their diverse applications.
The Geographic Layout of Metals on the Periodic Table
Metals overwhelmingly dominate the periodic table, occupying the vast majority of its space. Their location is not arbitrary; it reflects their shared electron configurations and resulting properties. You'll find metals predominantly residing in:
Groups 1-12 (Main-Group and Transition Metals):
This area is the heartland of metallic territory. Let's break it down:
-
Group 1 (Alkali Metals): These highly reactive metals are located in the far left column. Their single valence electron makes them extremely reactive with water and air, requiring special storage conditions. Examples include lithium (Li), sodium (Na), potassium (K), and cesium (Cs). The extreme reactivity decreases as you move down the group.
-
Group 2 (Alkaline Earth Metals): Situated to the right of the alkali metals, these metals are also reactive but less so than their Group 1 counterparts. They possess two valence electrons and are essential for various biological processes. Examples include beryllium (Be), magnesium (Mg), calcium (Ca), and barium (Ba).
-
Groups 3-12 (Transition Metals): This expansive block constitutes the majority of the metals on the periodic table. These elements are characterized by partially filled d orbitals, leading to variable oxidation states and a wide array of colorful compounds. Transition metals are known for their catalytic properties and are extensively used in industry and technology. Examples range from iron (Fe) and copper (Cu) to platinum (Pt) and gold (Au). The variation in properties within this block is enormous. For example, iron is essential for life and is a major component of steel, while platinum is used in catalytic converters and jewelry.
Lanthanides and Actinides:
These two series, located below the main body of the periodic table, represent inner transition metals. They are characterized by filling of the 4f and 5f orbitals, respectively.
-
Lanthanides (Rare Earth Metals): These 15 elements exhibit similar chemical properties, making their separation a significant challenge. They possess unique magnetic and luminescent properties, crucial for applications in modern electronics and lighting.
-
Actinides: This series comprises radioactive elements, many of which are synthetically produced. Their radioactive nature necessitates careful handling and specialized storage. Uranium (U) and plutonium (Pu) are notable examples, significantly used in nuclear energy and weapons technology.
Post-Transition Metals:
These metals, found adjacent to the transition metals, exhibit properties intermediate between transition metals and nonmetals. They tend to be less reactive than alkali and alkaline earth metals. Examples include aluminum (Al), tin (Sn), and lead (Pb). Aluminum's lightweight and corrosion-resistant properties make it ideal for construction and packaging, while tin is used in coatings to prevent corrosion. Lead, however, is increasingly restricted due to its toxicity.
Properties of Metals: A Consequence of Location
The location of metals on the periodic table directly influences their characteristic properties. These properties are the basis for their extensive applications in various industries.
Physical Properties:
-
Conductivity: Metals are excellent conductors of heat and electricity. This is due to the presence of delocalized electrons that can easily move throughout the metal lattice. This is why metals are used extensively in electrical wiring and heat exchangers.
-
Malleability and Ductility: Metals can be easily shaped (malleability) and drawn into wires (ductility). This is a direct consequence of the metallic bonding where atoms are held together by a sea of delocalized electrons. This allows layers of atoms to slide over one another without breaking the metallic bond.
-
Luster: Metals typically have a shiny or lustrous appearance. This is due to their ability to reflect light efficiently.
-
Density: Metals generally exhibit high densities, although there are exceptions. This is related to the close packing of atoms in the metallic lattice.
Chemical Properties:
-
Low Ionization Energies: Metals tend to have low ionization energies, meaning they readily lose electrons to form positive ions (cations). This is crucial in forming ionic compounds and participating in redox reactions.
-
Electropositivity: Metals are electropositive, meaning they have a tendency to lose electrons and become positively charged. This property is a consequence of their low electronegativity.
-
Reactivity: The reactivity of metals varies considerably across the periodic table. Alkali metals are the most reactive, while noble metals like gold and platinum are relatively unreactive. This reactivity is directly related to the ease with which they lose electrons.
Exceptions and Borderline Cases: Metalloids
While the majority of elements in the specified regions are definitively metals, there are some exceptions and borderline cases. These elements, known as metalloids, exhibit properties intermediate between metals and nonmetals. They are found along the "staircase" line that separates metals from nonmetals on the periodic table. Metalloids such as silicon (Si), germanium (Ge), and arsenic (As) possess some metallic properties like conductivity but also exhibit nonmetallic characteristics, such as their ability to form covalent bonds. Their properties are highly dependent on factors like temperature and pressure.
Applications of Metals: A Reflection of their Properties
The wide range of properties exhibited by metals has led to their widespread application in various fields.
Construction and Infrastructure:
Iron and steel alloys are fundamental materials in construction, forming the backbone of buildings, bridges, and vehicles. Aluminum’s lightweight and corrosion-resistant properties make it ideal for aerospace applications and building facades.
Electronics and Technology:
Copper is widely used in electrical wiring due to its excellent conductivity. Silicon, although a metalloid, is crucial in semiconductors and integrated circuits, the building blocks of modern electronics. Rare earth metals are essential components in magnets, lasers, and various electronic devices.
Transportation:
Aluminum, steel, and various alloys are extensively used in the automotive and aerospace industries. Their strength-to-weight ratio and corrosion resistance make them preferred choices.
Energy:
Metals play a crucial role in energy generation and storage. Uranium is used in nuclear power plants, while various metals are used in batteries and fuel cells.
Medicine and Biotechnology:
Metals have significant applications in medicine. Titanium's biocompatibility makes it ideal for implants and prosthetics. Platinum-based compounds are used in chemotherapy. Magnesium is an essential nutrient for the human body.
Catalysis:
Transition metals, especially platinum, palladium, and nickel, are extensively used as catalysts in various chemical processes. Their ability to facilitate chemical reactions makes them crucial in industrial production.
Conclusion: A Periodic Perspective on Metallic Abundance
The periodic table serves as a powerful tool for understanding the distribution and properties of elements. The overwhelming dominance of metals in specific regions highlights their importance in our world. From the highly reactive alkali metals to the inert noble metals, the diversity of metallic properties fuels innovation and drives technological advancements across numerous sectors. Understanding the periodic table’s organization allows us to anticipate and exploit the unique characteristics of metals, shaping our technological landscape and impacting various aspects of our lives. Further research into the nuanced properties and potential applications of metals continues to reveal new possibilities and expand our understanding of the periodic system's profound influence on our world.
Latest Posts
Latest Posts
-
What Is 40 Percent Of 1200
Apr 02, 2025
-
21 Is What Percent Of 25
Apr 02, 2025
-
An Organism That Eats Only Plants
Apr 02, 2025
-
What Is 1 To The 5th Power
Apr 02, 2025
-
Takes The Place Of A Noun
Apr 02, 2025
Related Post
Thank you for visiting our website which covers about Where On The Periodic Table Are Metals Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
