Where Is The Highest Electronegativity Found
listenit
Mar 24, 2025 · 5 min read
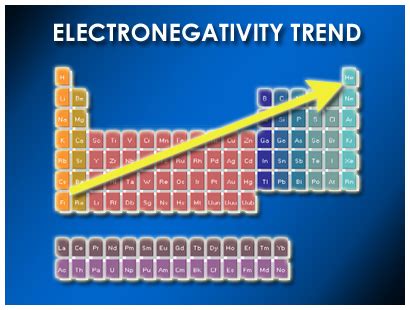
Table of Contents
Where is the Highest Electronegativity Found? Understanding Electronegativity Trends in the Periodic Table
Electronegativity, a fundamental concept in chemistry, describes an atom's ability to attract electrons towards itself within a chemical bond. Understanding electronegativity is crucial for predicting the nature of chemical bonds (ionic, covalent, or polar covalent), the polarity of molecules, and many other chemical properties. This article delves deep into the location of the highest electronegativity on the periodic table, exploring the trends, exceptions, and the underlying reasons behind these observations.
Understanding Electronegativity: A Closer Look
Before pinpointing the element with the highest electronegativity, let's solidify our understanding of the concept. Electronegativity isn't a directly measurable quantity like mass or charge. Instead, it's a relative property, meaning we compare the electronegativity of one element to another. Several scales exist to quantify electronegativity, the most common being the Pauling scale, developed by Linus Pauling. This scale assigns fluorine (F) a value of 4.0, the highest electronegativity value, serving as a benchmark for comparison.
Factors Influencing Electronegativity:
Several factors contribute to an atom's electronegativity:
- Nuclear Charge: A higher nuclear charge (more protons) strongly attracts electrons, increasing electronegativity.
- Atomic Radius: A smaller atomic radius means electrons are closer to the nucleus, experiencing a stronger attractive force, leading to higher electronegativity.
- Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus. Greater shielding reduces the effective nuclear charge experienced by outer electrons, lowering electronegativity.
Trends in Electronegativity Across the Periodic Table
Electronegativity demonstrates predictable trends across the periodic table:
1. Across a Period (Left to Right): Electronegativity generally increases as you move from left to right across a period. This is because the nuclear charge increases while the atomic radius generally decreases. The added protons pull the electrons more strongly towards the nucleus.
2. Down a Group (Top to Bottom): Electronegativity generally decreases as you move down a group. This is primarily due to the increasing atomic radius. The increased distance between the nucleus and the valence electrons reduces the attractive force, leading to lower electronegativity.
The Element with the Highest Electronegativity: Fluorine
Based on the Pauling scale, fluorine (F) holds the title of the most electronegative element, with a value of 4.0. Its position in the periodic table perfectly explains this high electronegativity:
- Small Atomic Radius: Fluorine is a small atom, placing its valence electrons relatively close to the nucleus. This results in a strong electrostatic attraction between the nucleus and the electrons.
- High Nuclear Charge: Compared to elements to its left in period 2, fluorine has a relatively high nuclear charge, further strengthening the pull on its valence electrons.
- Minimal Shielding: Having only two electron shells, fluorine experiences minimal shielding from inner electrons, allowing the nucleus to exert its full influence on the valence electrons.
Exceptions and Considerations
While the general trends are reliable, some exceptions and nuances exist:
- Noble Gases: Noble gases generally exhibit very low electronegativity due to their stable electron configurations. They rarely form chemical bonds, and thus, their electronegativity is often not considered in the same context as other elements.
- Transition Metals: Predicting electronegativity for transition metals is more complex due to variations in d-electron shielding and multiple oxidation states. The trends are less clear-cut than in the main group elements.
- Other Electronegativity Scales: While the Pauling scale is widely used, other scales exist (e.g., Mulliken, Allred-Rochow), which may yield slightly different electronegativity values. However, the overall trend remains consistent: fluorine consistently tops the list.
Applications of Electronegativity: Understanding Chemical Bonding
The concept of electronegativity is fundamental in understanding the nature of chemical bonds:
-
Ionic Bonds: When the electronegativity difference between two atoms is large (typically greater than 1.7 on the Pauling scale), electrons are effectively transferred from the less electronegative atom to the more electronegative atom, forming ions and an ionic bond. For example, NaCl (sodium chloride) has a large electronegativity difference, resulting in an ionic bond.
-
Covalent Bonds: When the electronegativity difference between two atoms is small (typically less than 0.5), electrons are shared more or less equally between the atoms, leading to a nonpolar covalent bond. For example, the bond in a diatomic oxygen molecule (O2) is nonpolar covalent.
-
Polar Covalent Bonds: When the electronegativity difference falls between 0.5 and 1.7, electrons are shared unequally, resulting in a polar covalent bond. The more electronegative atom carries a partial negative charge (δ-), while the less electronegative atom carries a partial positive charge (δ+). Water (H2O) is a classic example of a molecule with polar covalent bonds.
The Significance of Fluorine's High Electronegativity
Fluorine's exceptional electronegativity has significant implications across various scientific fields:
-
Chemistry: Fluorine's high electronegativity drives the formation of strong and stable chemical bonds, making fluorinated compounds prevalent in diverse applications.
-
Materials Science: Fluorine's influence on the properties of materials is vast. For instance, fluoropolymers (like Teflon) exhibit exceptional chemical resistance and low friction due to the strong C-F bonds.
-
Biology: Fluorine is incorporated into some pharmaceuticals to enhance their potency or stability. However, it also plays an important role in the toxicity of certain compounds.
-
Medicine: Fluorine's unique properties are exploited in medicinal chemistry for developing drugs with improved efficacy and bioavailability. Examples include fluoroquinolone antibiotics and fluorinated anesthetic agents.
Conclusion: The Reign of Fluorine
Fluorine's position at the apex of electronegativity on the periodic table underscores the importance of understanding periodic trends. The interplay between nuclear charge, atomic radius, and shielding effects ultimately governs an atom's ability to attract electrons. This ability, quantified by electronegativity, dictates the nature of chemical bonds, influencing the properties of countless substances across various disciplines, from materials science to medicine. Further research continues to explore the nuances of electronegativity and its wide-ranging applications. While the general trend of increasing electronegativity across a period and decreasing down a group holds, the intricacies of electron configurations and orbital interactions need to be considered for a more comprehensive understanding. This detailed exploration sheds light on why fluorine reigns supreme as the most electronegative element.
Latest Posts
Latest Posts
-
What Is The Electron Configuration Of N
Mar 26, 2025
-
How Long Is 9 Feet In Meters
Mar 26, 2025
-
What Is The Greatest Common Factor Of 48 And 84
Mar 26, 2025
-
What Is 17 Out Of 20 As A Percentage
Mar 26, 2025
-
What Percent Of 20 Is 17
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about Where Is The Highest Electronegativity Found . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
