What Is The Electron Configuration Of N
listenit
Mar 26, 2025 · 5 min read
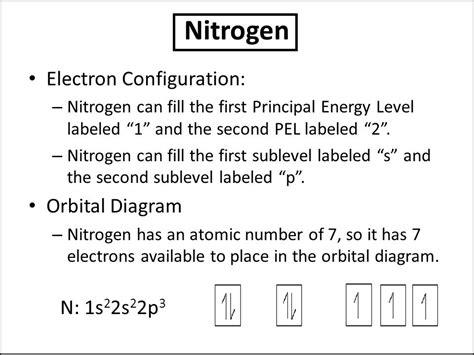
Table of Contents
What is the Electron Configuration of Nitrogen? Understanding Atomic Structure and its Implications
Nitrogen, a crucial element for life as we know it, holds a fascinating position in the periodic table. Its atomic structure, specifically its electron configuration, dictates its chemical properties and reactivity. Understanding this configuration is key to comprehending nitrogen's role in various chemical processes, from forming the atmosphere to its importance in biological molecules. This comprehensive guide delves deep into the electron configuration of nitrogen, explaining its derivation, significance, and implications.
Understanding Electron Configuration
Before we delve into nitrogen's specific configuration, let's establish a foundational understanding of what electron configuration means. An electron configuration describes how electrons are distributed among the various energy levels and sublevels within an atom. This distribution follows specific rules governed by quantum mechanics, ensuring stability and predictability in atomic behavior.
Key Principles Governing Electron Configuration
Several fundamental principles guide the arrangement of electrons within an atom:
-
Aufbau Principle: Electrons fill the lowest energy levels first. This is analogous to filling a container from the bottom up; you wouldn't start filling the top until the bottom is full.
-
Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins. Think of it like a two-person room; you can't fit more than two people in, and they need to be oriented differently.
-
Hund's Rule: Electrons will individually occupy each orbital within a subshell before pairing up. This minimizes electron-electron repulsion, making the atom more stable. It's like choosing separate seats on a bus before doubling up.
These principles, combined with the understanding of atomic orbitals (s, p, d, f), allow us to predict and understand the electron configuration of any element.
Deriving the Electron Configuration of Nitrogen (N)
Nitrogen (N) has an atomic number of 7, meaning it has 7 protons and 7 electrons in a neutral atom. To determine its electron configuration, we'll systematically fill the orbitals according to the principles mentioned above:
-
The first energy level (n=1) contains only the 1s subshell. This subshell can hold a maximum of two electrons. Therefore, the first two electrons of nitrogen fill the 1s orbital: 1s².
-
The second energy level (n=2) contains the 2s and 2p subshells. The 2s subshell can also hold two electrons, so the next two electrons fill this orbital: 2s².
-
The 2p subshell has three orbitals (2px, 2py, 2pz), each capable of holding two electrons. Nitrogen has three remaining electrons. According to Hund's rule, these electrons will individually occupy each of the 2p orbitals before pairing up. This results in: 2p³.
Therefore, the complete electron configuration of nitrogen is 1s²2s²2p³.
Visualizing Nitrogen's Electron Configuration
It's often helpful to visualize the electron configuration. We can represent it using orbital diagrams:
1s: ↑↓
2s: ↑↓
2p: ↑ ↑ ↑ (Each arrow represents an electron with a specific spin)
This diagram clearly shows the two electrons in the 1s orbital, two electrons in the 2s orbital, and three unpaired electrons in the three 2p orbitals.
Significance of Nitrogen's Electron Configuration
Nitrogen's electron configuration has profound implications for its chemical behavior and reactivity:
-
Valence Electrons: The outermost electrons, those in the 2s and 2p subshells, are called valence electrons. Nitrogen has five valence electrons (2s²2p³). These electrons are crucial in forming chemical bonds.
-
Chemical Bonding: Nitrogen's five valence electrons allow it to form a variety of covalent bonds. It frequently forms three covalent bonds, as seen in ammonia (NH₃), achieving a stable octet (eight electrons in its valence shell). This stability is a cornerstone of its chemical reactivity.
-
Triple Bond Formation: Nitrogen is famously known for its ability to form a very strong triple bond with another nitrogen atom to form N₂ (dinitrogen), the major component of Earth's atmosphere. The triple bond is incredibly stable due to the strong attraction between the nuclei and shared electrons. This extraordinary stability explains why atmospheric nitrogen is relatively unreactive. Breaking this triple bond requires a significant amount of energy.
-
Biological Significance: Nitrogen's ability to form strong bonds with other elements makes it an essential component of many biologically important molecules, including amino acids (building blocks of proteins), nucleic acids (DNA and RNA), and chlorophyll (vital for photosynthesis). The nitrogen cycle, which involves the transformation of nitrogen between different chemical forms, is crucial for sustaining life on Earth.
-
Industrial Applications: The industrial production of ammonia via the Haber-Bosch process relies heavily on manipulating nitrogen's reactivity to create a crucial fertilizer for agriculture. This process significantly impacts global food production.
Beyond the Basics: Excited States and Ionization
While the ground-state electron configuration (1s²2s²2p³) describes nitrogen under normal conditions, it can also exist in excited states. In an excited state, one or more electrons jump to higher energy levels. This is typically a temporary state, and the electron will eventually return to its ground state, often emitting energy in the form of light.
Ionization involves the removal of an electron from an atom. Removing an electron from nitrogen requires energy, as it disrupts the stable electron configuration. The first ionization energy is the energy required to remove the first electron, and subsequent ionization energies are needed to remove additional electrons. The increasing ionization energies reflect the increasing difficulty in removing electrons as you approach a noble gas configuration.
Conclusion: The Importance of Understanding Electron Configuration
The electron configuration of nitrogen (1s²2s²2p³) is not merely a set of numbers and letters; it's a key to unlocking the element's properties, reactivity, and fundamental role in the natural world. Understanding this configuration allows us to comprehend its chemical behavior, biological significance, and industrial applications. From forming the atmosphere's stable diatomic nitrogen to its vital role in biological molecules and fertilizers, nitrogen's unique electronic structure is crucial to understanding a wide range of scientific phenomena. This fundamental understanding paves the way for further exploration in chemistry, biology, and material science, highlighting the importance of mastering basic atomic concepts. The seemingly simple electron configuration is a gateway to a world of complex and fascinating chemical interactions.
Latest Posts
Latest Posts
-
What Is The Gcf Of 45 And 36
Mar 29, 2025
-
What Number Is 45 Of 90
Mar 29, 2025
-
What Is 2 5 As A Decimal
Mar 29, 2025
-
18 As A Percentage Of 60
Mar 29, 2025
-
Is Hydrogen Gas At Room Temperature
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of N . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
