Where Is Energy Stored In Atp
listenit
Mar 19, 2025 · 5 min read
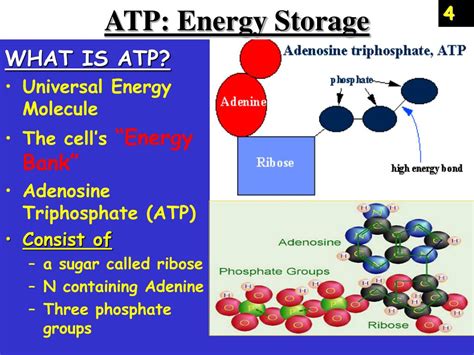
Table of Contents
Where is Energy Stored in ATP? Unraveling the Secrets of Cellular Energy
ATP, or adenosine triphosphate, is the fundamental energy currency of all living cells. Understanding where this energy is stored within the ATP molecule is crucial to grasping the very basis of life's processes. This article delves deep into the intricacies of ATP's structure and the precise location of its stored energy, exploring the chemical bonds and the mechanisms involved in energy release and utilization.
The Structure of ATP: A Molecular Powerhouse
Before understanding where energy is stored, we must first examine ATP's structure. ATP is a nucleotide composed of three main components:
- Adenine: A nitrogenous base, a crucial component of DNA and RNA.
- Ribose: A five-carbon sugar, forming the backbone of the molecule.
- Triphosphate group: Three phosphate groups linked together, the key to ATP's energy storage capacity. This triphosphate chain is where the magic happens.
It's the high-energy phosphate bonds within this triphosphate tail that hold the key to ATP's energy-storing capabilities. These are not ordinary chemical bonds; they are phosphoanhydride bonds, characterized by their high energy content.
High-Energy Phosphate Bonds: The Secret Sauce
The phosphate groups in ATP are negatively charged. Packing three negatively charged phosphate groups together in close proximity creates significant electrostatic repulsion. This repulsion stores a substantial amount of potential energy, much like a tightly wound spring. This is why these bonds are referred to as high-energy phosphate bonds. They are not stronger than other bonds, but their inherent instability makes them readily available for energy release upon hydrolysis.
Hydrolysis of ATP: Releasing the Stored Energy
The energy stored in ATP is released through a process called hydrolysis. Hydrolysis involves the breaking of a phosphoanhydride bond by the addition of a water molecule. This process typically involves the removal of the terminal phosphate group, converting ATP into adenosine diphosphate (ADP) and inorganic phosphate (Pi).
The Energetics of ATP Hydrolysis
The hydrolysis of ATP is an exergonic reaction, meaning it releases energy. The standard free energy change (ΔG°) for the hydrolysis of ATP to ADP and Pi is approximately -30.5 kJ/mol under standard conditions. This negative ΔG° indicates that the reaction is spontaneous and releases energy that can be harnessed to drive other cellular processes.
However, it's crucial to understand that the actual free energy change (ΔG) can vary depending on cellular conditions, including the concentrations of ATP, ADP, and Pi. The cell meticulously regulates these concentrations to control the energy released during ATP hydrolysis.
How Cells Utilize ATP's Energy: Coupling Reactions
The energy released during ATP hydrolysis is not directly used to power cellular processes. Instead, it's used to drive other reactions that would otherwise be endergonic (require energy input) through a process called energy coupling.
Coupling Exergonic and Endergonic Reactions
The cell cleverly couples the exergonic hydrolysis of ATP with the endergonic reactions it needs to perform. This coupling allows the energy released from ATP hydrolysis to overcome the energy barrier of the endergonic reaction, driving it forward.
Examples of processes powered by ATP hydrolysis include:
- Muscle contraction: The interaction of actin and myosin filaments requires ATP hydrolysis to generate the necessary force for movement.
- Active transport: Moving molecules against their concentration gradient, as in the sodium-potassium pump, necessitates ATP hydrolysis.
- Biosynthesis: Building complex molecules from simpler precursors, such as protein synthesis, requires energy derived from ATP hydrolysis.
- Nerve impulse transmission: The transmission of nerve impulses involves changes in ion concentrations across the neuronal membrane, powered by ATP-driven ion pumps.
- DNA replication and transcription: The complex processes of replicating and transcribing DNA rely heavily on the energy provided by ATP hydrolysis.
Beyond ATP Hydrolysis: Other Energy Release Pathways
While hydrolysis of the terminal phosphate is the most common and well-studied pathway for energy release from ATP, other pathways also exist:
- Hydrolysis of the second phosphate: Removal of the second phosphate group converts ADP to adenosine monophosphate (AMP) and releases additional energy, albeit less than the first hydrolysis.
- Phosphorylation: ATP can directly transfer its phosphate group to other molecules, a process called phosphorylation. This phosphorylation can activate or modify the target molecule, altering its activity. This is crucial in signaling pathways and enzymatic regulation.
ATP Synthesis: Regenerating the Energy Currency
The supply of ATP is constantly replenished through several metabolic pathways, primarily cellular respiration (in aerobic organisms) and fermentation (in anaerobic organisms). These processes generate ATP from ADP and Pi, storing energy in the high-energy phosphate bonds for future use.
Cellular Respiration: The Major ATP Generator
Cellular respiration is the most efficient method for ATP synthesis. It involves a series of redox reactions where electrons are passed along an electron transport chain, generating a proton gradient that drives ATP synthase, the enzyme responsible for synthesizing ATP.
Fermentation: An Anaerobic Alternative
Fermentation is an anaerobic process that produces ATP without the involvement of oxygen. It's less efficient than cellular respiration but provides a vital energy source in oxygen-deprived conditions.
Conclusion: ATP – The Heart of Cellular Energy
The energy stored in ATP is not located in a single specific place but rather is distributed across the molecule. The high-energy phosphate bonds, particularly the terminal phosphoanhydride bond, are the primary sites of energy storage. The electrostatic repulsion between the negatively charged phosphate groups and the inherent instability of these bonds make them readily available for hydrolysis, releasing energy that drives a vast array of crucial cellular processes. Understanding the structure and function of ATP is essential to comprehending the fundamental mechanisms that power life itself. The intricate interplay between ATP hydrolysis, energy coupling, and ATP synthesis ensures a continuous flow of energy within the cell, maintaining its vital functions. This elegant mechanism underlines the remarkable efficiency and sophistication of cellular processes. The study of ATP continues to reveal further intricacies, highlighting its central role in various biological functions and offering potential avenues for therapeutic interventions in diseases associated with metabolic dysfunction.
Latest Posts
Latest Posts
-
How Much Is 1 4 Lb
Mar 20, 2025
-
How Many Quarts In 40 Gallons
Mar 20, 2025
-
How Many Atoms Are In Carbon
Mar 20, 2025
-
In Which Organelle Does Photosynthesis Take Place
Mar 20, 2025
-
What Percentage Of 55 Is 34
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about Where Is Energy Stored In Atp . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
