How Many Atoms Are In Carbon
listenit
Mar 20, 2025 · 6 min read
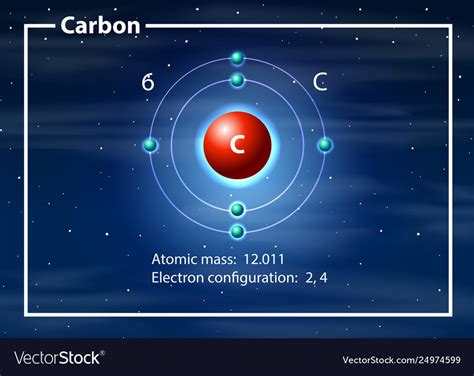
Table of Contents
Delving into the Atomic Composition of Carbon: How Many Atoms Are There?
The question, "How many atoms are in carbon?" is deceptively simple. It requires unpacking several crucial concepts in chemistry and physics before we can arrive at a meaningful answer. The short answer is: it depends. It depends on which carbon we're talking about – a single carbon atom, a mole of carbon, a diamond, a graphite pencil – the amount of carbon dictates the number of atoms involved. Let's explore the intricacies of this question in detail.
Understanding the Basics: Atoms and Moles
At the heart of this question lies the atom, the fundamental building block of matter. Carbon, denoted by the symbol C and atomic number 6, is a chemical element. This means all carbon atoms possess six protons in their nucleus, defining their identity. The number of neutrons can vary, leading to different isotopes of carbon (like Carbon-12, Carbon-13, and Carbon-14), but they are all still carbon atoms.
The concept of a mole is crucial when dealing with large quantities of atoms. A mole is a unit of measurement in chemistry representing Avogadro's number (approximately 6.022 x 10<sup>23</sup>) of particles, whether those particles are atoms, molecules, or ions. This enormous number reflects the incredibly small size of atoms.
How Many Atoms in a Single Carbon Atom?
This might seem trivial, but it's important to establish a baseline. A single carbon atom contains, by definition, one carbon atom. This seemingly simple statement highlights the fundamental nature of atoms as indivisible units in chemical reactions (at least, under normal conditions).
How Many Atoms in a Mole of Carbon?
This is where Avogadro's number comes into play. One mole of carbon contains approximately 6.022 x 10<sup>23</sup> carbon atoms. This is a direct application of the definition of a mole. This vast number underscores the scale at which chemical reactions occur. It's important to remember that this is an average number, accounting for the natural abundance of different carbon isotopes.
Carbon's Diverse Forms: Allotropes and the Atom Count
Carbon exhibits a remarkable property known as allotropy, meaning it can exist in different forms with distinct physical and chemical properties despite being composed of the same atoms. The most well-known allotropes are diamond and graphite. Let's delve into how this affects the answer to our original question.
Diamonds: A Precise Atomic Structure
Diamonds are renowned for their hardness and brilliance. Their atomic structure is a three-dimensional network of carbon atoms arranged in a strong, tetrahedral lattice. Each carbon atom is covalently bonded to four other carbon atoms, creating a rigid and highly stable structure. Determining the exact number of atoms in a diamond depends entirely on the diamond's size and mass. A larger diamond will naturally contain a greater number of carbon atoms. There isn't a single answer; it varies significantly depending on the diamond's dimensions. One would need to know the mass of the diamond and then use the molar mass of carbon (12.01 g/mol) and Avogadro's number to calculate the approximate number of carbon atoms.
Graphite: Layered Carbon
Graphite, in contrast to diamond, is a soft, slippery material used in pencils. Its structure consists of layers of carbon atoms arranged in a hexagonal lattice. While the strong bonds within each layer exist, the bonds between layers are much weaker. This accounts for graphite's softness and ability to leave marks on paper. Similar to diamonds, the number of carbon atoms in a piece of graphite depends directly on its mass and dimensions. Again, a mass-to-atom calculation using molar mass and Avogadro's number would be needed to determine the precise number.
Other Carbon Allotropes: Fullerenes, Carbon Nanotubes, and Amorphous Carbon
Beyond diamonds and graphite, carbon exists in other fascinating allotropes, including fullerenes (like buckminsterfullerene, or "buckyballs"), carbon nanotubes, and amorphous carbon. Each of these forms has unique atomic arrangements, making the calculation of the total number of atoms dependent on the size and specific structure of the material. For example, a buckyball (C60) contains exactly 60 carbon atoms. However, the number of atoms in a carbon nanotube or amorphous carbon sample would necessitate sophisticated analytical techniques and mass measurements.
Calculating the Number of Atoms in a Given Mass of Carbon
For a given mass of carbon, irrespective of its allotropic form (assuming pure carbon), we can calculate the approximate number of atoms present. The process involves the following steps:
- Determine the mass (m) of the carbon sample: This must be measured in grams.
- Find the molar mass (M) of carbon: The molar mass of carbon is approximately 12.01 grams per mole (g/mol). This accounts for the natural abundance of its isotopes.
- Calculate the number of moles (n): Use the formula: n = m/M
- Calculate the number of atoms (N): Use Avogadro's number (N<sub>A</sub> ≈ 6.022 x 10<sup>23</sup> atoms/mol): N = n x N<sub>A</sub>
Example:
Let's say we have a 1-gram sample of pure carbon.
- m = 1 g
- M = 12.01 g/mol
- n = 1 g / 12.01 g/mol ≈ 0.0833 mol
- N ≈ 0.0833 mol x 6.022 x 10<sup>23</sup> atoms/mol ≈ 5.01 x 10<sup>22</sup> atoms
Therefore, a 1-gram sample of pure carbon contains approximately 5.01 x 10<sup>22</sup> carbon atoms. Remember that this is an approximation, accounting for the average molar mass of naturally occurring carbon.
Beyond the Simple Question: Isotopes and Impurities
The calculations above assume pure carbon consisting of a typical isotopic mixture. However, the presence of carbon isotopes (<sup>12</sup>C, <sup>13</sup>C, <sup>14</sup>C) and impurities can slightly alter the final atom count. <sup>14</sup>C, for example, is radioactive and exists in trace amounts. Accounting for these variations requires more complex calculations and specialized analytical techniques. These subtleties remind us that even the simplest question in chemistry can reveal layers of complexity.
Conclusion: The Elusive Answer
The initial question, "How many atoms are in carbon?" doesn't have a single definitive answer. It depends entirely on the amount and form of carbon being considered. Whether it's a single atom, a mole, a diamond, or graphite, the number of carbon atoms varies significantly. Understanding the concepts of atoms, moles, Avogadro's number, and carbon's allotropic forms is key to appreciating the nuances of this seemingly straightforward inquiry. The calculations presented provide a framework for estimating atom counts in various carbon samples, highlighting the vast scale of the atomic world and the complexity of matter at its fundamental level. Further considerations, like isotopic variations and impurities, illustrate that even seemingly simple questions can lead to deeper explorations in the world of chemistry and physics.
Latest Posts
Latest Posts
-
What Do Roman Numerals Mean In Chemistry
Mar 20, 2025
-
What Is 5 And 1 8 As A Decimal
Mar 20, 2025
-
How Many Electrons Can The 4th Energy Level Hold
Mar 20, 2025
-
What Is The Molar Mass Of Iron
Mar 20, 2025
-
How To Punctuate The Title Of A Movie
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Atoms Are In Carbon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
