How Many Electrons Can The 4th Energy Level Hold
listenit
Mar 20, 2025 · 6 min read
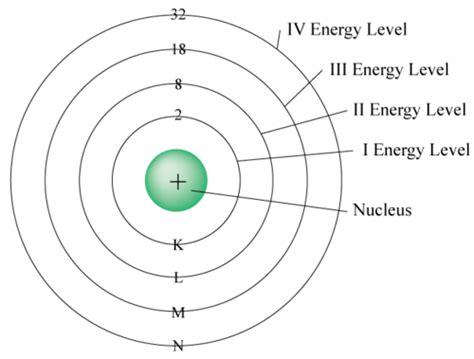
Table of Contents
How Many Electrons Can the 4th Energy Level Hold? A Deep Dive into Atomic Structure
Understanding the electron configuration of atoms is fundamental to comprehending chemistry and physics. A crucial aspect of this understanding is knowing how many electrons each energy level can hold. This article delves into the specifics of the fourth energy level, exploring the underlying principles governing electron occupancy and its implications for atomic behavior.
Understanding Electron Shells and Subshells
Before we tackle the fourth energy level, let's review the basics of atomic structure. Electrons, negatively charged particles, orbit the nucleus (containing positively charged protons and neutral neutrons) in distinct energy levels, often visualized as shells. These shells are not physical orbits, but rather represent regions of space where there's a high probability of finding an electron.
Each energy level is associated with a specific principal quantum number (n), where n = 1 represents the first energy level (closest to the nucleus), n = 2 the second, and so on. The higher the value of n, the farther the electrons are from the nucleus and the higher their energy.
Within each energy level (except for n=1), electrons are further organized into subshells. These subshells are designated by letters: s, p, d, and f. Each subshell can hold a specific number of electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The number of subshells within a given energy level is equal to the value of the principal quantum number (n).
The Fourth Energy Level (n=4): A Detailed Examination
Now, let's focus on the fourth energy level (n=4). Since n=4, it contains four subshells: 4s, 4p, 4d, and 4f. To determine the total number of electrons this level can hold, we simply sum the maximum number of electrons each subshell can accommodate:
- 4s subshell: 2 electrons
- 4p subshell: 6 electrons
- 4d subshell: 10 electrons
- 4f subshell: 14 electrons
Total electrons in the 4th energy level: 2 + 6 + 10 + 14 = 32 electrons
Therefore, the fourth energy level can hold a maximum of 32 electrons.
The Aufbau Principle and Electron Filling
The Aufbau principle dictates the order in which electrons fill the energy levels and subshells. Electrons fill the lowest energy levels first before moving to higher energy levels. While generally following this order, there are exceptions due to subtle energy differences between subshells. The order of filling is approximately:
1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p…
Note that the 4s subshell fills before the 3d subshell, even though the 3d subshell is part of the third energy level. This is due to the slight energy difference between these subshells.
Implications for Chemical Properties
The electron configuration, particularly the number of electrons in the outermost energy level (valence electrons), significantly influences an atom's chemical properties. Atoms tend to react in ways that achieve a stable electron configuration, often by gaining, losing, or sharing electrons to fill their outermost shell or achieve a noble gas configuration. Elements with filled outermost shells are generally unreactive (noble gases).
The fourth energy level's capacity to hold 32 electrons means elements with electrons in this level exhibit a wide range of chemical behaviors. The transition metals, lanthanides, and actinides all have electrons occupying the 4th energy level (specifically, the 3d, 4f, and 5f subshells respectively), showcasing the diverse reactivity of these elements.
Orbital Shapes and Electron Probability
Understanding the electron configuration requires going beyond simply stating the number of electrons. It's also important to grasp the spatial distribution of electrons within the subshells. Each subshell is composed of atomic orbitals, regions of space where there is a high probability of finding an electron. These orbitals have characteristic shapes:
- s orbitals: Spherical shape
- p orbitals: Dumbbell shape (three p orbitals oriented along the x, y, and z axes)
- d orbitals: More complex shapes (five d orbitals)
- f orbitals: Even more complex shapes (seven f orbitals)
Each orbital can hold a maximum of two electrons, following the Pauli Exclusion Principle, which states that no two electrons in an atom can have the same set of four quantum numbers (n, l, ml, and ms).
Beyond the Fourth Energy Level
While we've focused on the fourth energy level, it's important to remember that higher energy levels exist. The fifth energy level (n=5) can hold even more electrons (50), following the same pattern of subshells (5s, 5p, 5d, 5f, 5g – although the 5g subshell is not filled in any known naturally occurring elements). The number of electrons a given energy level can hold continues to increase with increasing principal quantum number (n).
The general formula for calculating the maximum number of electrons an energy level can hold is 2n², where n is the principal quantum number. For n=4, this gives 2(4)² = 32, consistent with our earlier calculation.
Exceptions and Refinements to the Rules
It is crucial to note that the Aufbau principle and the simple 2n² formula provide a good approximation but aren't always perfectly accurate. Electron-electron repulsions and other subtle quantum mechanical effects can lead to slight variations in the order of electron filling. Some elements exhibit exceptions to the expected electron configurations, requiring a more nuanced understanding of atomic structure.
Practical Applications and Further Research
Understanding electron configurations has far-reaching applications across various scientific fields. It's crucial for:
- Predicting chemical reactivity: Knowing the number of valence electrons helps predict how atoms will interact to form molecules and compounds.
- Spectroscopy: Analyzing the absorption and emission spectra of atoms provides insights into their electron configurations and energy levels.
- Materials science: Designing new materials with specific properties often involves manipulating the electron configurations of constituent atoms.
- Nuclear physics: Understanding the stability of atomic nuclei is closely linked to the electron configurations of the surrounding electrons.
The study of atomic structure and electron configurations is an ongoing area of research. Advances in theoretical and experimental techniques continuously refine our understanding of the complexities of electron behavior within atoms.
Conclusion
The fourth energy level can hold a maximum of 32 electrons, distributed across its four subshells (4s, 4p, 4d, and 4f). This capacity significantly impacts the chemical and physical properties of elements with electrons in this level. Understanding the principles governing electron occupancy, including the Aufbau principle and Pauli exclusion principle, is vital for comprehending atomic structure and its implications for various scientific disciplines. While the 2n² formula provides a useful approximation, exceptions and subtle nuances in electron configurations require a deeper dive into quantum mechanics. Continued research further refines our knowledge, expanding our understanding of the intricate world of atomic structure.
Latest Posts
Latest Posts
-
Common Denominator Of 7 And 9
Mar 20, 2025
-
Least Common Factor Of 36 And 45
Mar 20, 2025
-
What Unit Is Acceleration Measured In
Mar 20, 2025
-
Which Describes An Effect Of Prohibition
Mar 20, 2025
-
What Are The Factors Of 41
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about How Many Electrons Can The 4th Energy Level Hold . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
