When Pressure Increases Then The Volume Must
listenit
Mar 24, 2025 · 6 min read
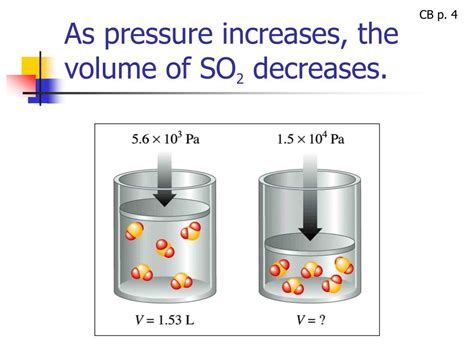
Table of Contents
When Pressure Increases, Then the Volume Must... Decrease! Understanding Boyle's Law
The relationship between pressure and volume has fascinated scientists for centuries. Understanding how these two properties interact is crucial in numerous fields, from designing scuba diving equipment to predicting weather patterns. This comprehensive article delves into the fundamental principle governing this relationship: Boyle's Law, exploring its implications and applications in various contexts.
What is Boyle's Law?
Boyle's Law, also known as the Boyle-Mariotte law, is a fundamental gas law stating that the absolute pressure and volume of a given mass of ideal gas are inversely proportional, provided the temperature and the amount of gas remain constant. In simpler terms, if you increase the pressure on a gas, its volume will decrease proportionally, and vice-versa. This inverse relationship is mathematically expressed as:
P₁V₁ = P₂V₂
Where:
- P₁ represents the initial pressure
- V₁ represents the initial volume
- P₂ represents the final pressure
- V₂ represents the final volume
This equation allows us to predict the changes in volume resulting from pressure changes, or vice-versa, under constant temperature and gas quantity conditions. Let's delve deeper into the implications of this law.
Understanding the Inverse Relationship
The core concept of Boyle's Law lies in its inverse proportionality. Imagine a gas contained within a sealed container. The gas molecules are constantly moving and colliding with the container walls, exerting pressure. If we reduce the container's volume, we confine the gas molecules into a smaller space. This leads to:
- Increased Collision Frequency: The molecules collide more frequently with each other and the container walls.
- Increased Collision Force: The collisions become more forceful due to the reduced space.
- Increased Pressure: The overall pressure exerted by the gas on the container increases proportionally to the decrease in volume.
Conversely, if we increase the container's volume, the gas molecules have more space to move around. This results in:
- Decreased Collision Frequency: Molecules collide less frequently.
- Decreased Collision Force: Collisions become less forceful.
- Decreased Pressure: The overall pressure exerted by the gas decreases proportionally to the increase in volume.
This dynamic interplay between pressure and volume is crucial for understanding the behavior of gases under varying conditions.
Ideal Gas Assumptions and Limitations
It's important to note that Boyle's Law is based on the ideal gas law, which assumes that:
- Gas molecules have negligible volume: This means the volume occupied by the gas molecules themselves is insignificant compared to the total volume of the container.
- There are no intermolecular forces: Gas molecules are assumed to not interact with each other, except during elastic collisions.
- Collisions are perfectly elastic: No energy is lost during collisions between gas molecules or between gas molecules and container walls.
These assumptions are not perfectly accurate for real gases, especially at high pressures and low temperatures where intermolecular forces become significant. However, Boyle's Law provides a reasonably accurate approximation for many real gases under moderate conditions. Deviations from ideal behavior are typically observed under extreme conditions.
Real-World Applications of Boyle's Law
Boyle's Law has wide-ranging applications across numerous scientific and engineering disciplines. Here are some examples:
1. Scuba Diving: Divers must understand Boyle's Law to manage the pressure changes experienced at different depths. As divers descend, the pressure increases, causing the air in their lungs to compress. Failure to account for this compression can lead to serious injuries. Conversely, as divers ascend, the pressure decreases, and the air in their lungs expands, potentially causing lung injuries if ascent is too rapid. Proper breathing techniques and equipment design are crucial for mitigating these risks.
2. Meteorology: Boyle's Law plays a vital role in understanding weather patterns. Atmospheric pressure changes affect air volume and movement. Variations in pressure influence the formation of clouds, storms, and wind patterns. Meteorologists utilize this knowledge to predict weather events.
3. Pneumatic Systems: Boyle's Law is fundamental to the design and operation of pneumatic systems, which use compressed air to power machinery and tools. The principle governs how air compressors work and how pneumatic actuators function. Understanding pressure and volume relationships is critical for optimizing pneumatic system performance and efficiency.
4. Medical Applications: Boyle's Law principles are applied in various medical devices and procedures. For instance, the function of syringes, ventilation systems, and other medical equipment relies on the relationship between pressure and volume.
5. Industrial Processes: Many industrial processes involve the compression or expansion of gases, and Boyle's Law is essential for controlling and optimizing these processes. Examples include the production of chemicals, refining of petroleum, and manufacturing of various products.
6. Respiratory System: The human respiratory system provides another compelling example of Boyle's Law in action. Breathing involves the expansion and contraction of the lungs, altering their volume and thus the pressure within. This pressure difference allows for inhalation and exhalation.
Beyond Boyle's Law: Considering Temperature and the Ideal Gas Law
While Boyle's Law provides a valuable framework for understanding the pressure-volume relationship at constant temperature, it's essential to acknowledge its limitations when temperature changes. The Ideal Gas Law offers a more comprehensive description of gas behavior by incorporating temperature:
PV = nRT
Where:
- P is the absolute pressure
- V is the volume
- n is the amount of gas (in moles)
- R is the ideal gas constant
- T is the absolute temperature
The Ideal Gas Law accounts for the combined effects of pressure, volume, temperature, and the amount of gas. Boyle's Law can be considered a special case of the Ideal Gas Law where temperature and the number of moles remain constant.
Exploring Deviations from Ideal Gas Behavior
Real gases deviate from the ideal gas law under certain conditions. At high pressures, gas molecules occupy a significant portion of the container's volume, rendering the assumption of negligible molecular volume invalid. At low temperatures, intermolecular forces become significant, affecting the behavior of the gas molecules.
The van der Waals equation is a more sophisticated model that accounts for these deviations by incorporating correction terms for intermolecular forces and molecular volume:
(P + a(n/V)²)(V - nb) = nRT
Where:
- a and b are van der Waals constants that depend on the specific gas.
This equation provides a more accurate description of real gas behavior, especially under conditions where Boyle's Law might not be sufficient.
Experimental Verification of Boyle's Law
The validity of Boyle's Law has been extensively verified through numerous experiments. Simple experiments can be performed using a syringe and a pressure gauge to demonstrate the inverse relationship between pressure and volume. By changing the volume of the syringe and observing the corresponding pressure changes, one can visually confirm the inverse relationship predicted by Boyle's Law.
More sophisticated experiments using precise measurement equipment can further confirm the validity of the law under various conditions. These experiments help refine our understanding of gas behavior and its deviations from ideal conditions.
Conclusion: The Enduring Significance of Boyle's Law
Boyle's Law remains a cornerstone of our understanding of gas behavior. While it relies on ideal gas assumptions that are not perfectly true for all gases under all conditions, it provides a robust and practical framework for predicting and understanding the relationship between pressure and volume for many real-world applications. The inverse relationship between pressure and volume, articulated by this fundamental law, is crucial for designing and operating countless technologies and understanding diverse natural phenomena. Coupled with the Ideal Gas Law and models like the van der Waals equation, Boyle's Law provides a powerful set of tools for accurately predicting and explaining the behavior of gases in various contexts. Its enduring significance in science and engineering underscores its fundamental importance.
Latest Posts
Latest Posts
-
How To Find Z Score For Percentile
Mar 26, 2025
-
What Is The Electron Configuration Of N
Mar 26, 2025
-
How Long Is 9 Feet In Meters
Mar 26, 2025
-
What Is The Greatest Common Factor Of 48 And 84
Mar 26, 2025
-
What Is 17 Out Of 20 As A Percentage
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about When Pressure Increases Then The Volume Must . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
