When An Ionic Bond Forms Electrons Are
listenit
Mar 18, 2025 · 6 min read
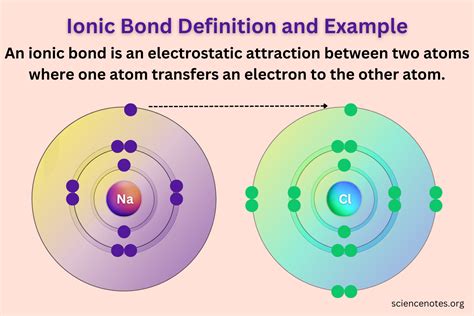
Table of Contents
When an Ionic Bond Forms, Electrons Are…Transferred! Understanding Ionic Bonding
Ionic bonds are fundamental to chemistry, forming the basis of countless compounds and significantly impacting the properties of materials we encounter daily. Understanding how these bonds form is crucial to grasping chemical reactions and the behavior of matter. This article delves deep into the process of ionic bond formation, explaining precisely what happens to electrons when this type of bond is created. We'll explore the concepts of electronegativity, ionization energy, and lattice energy, providing a comprehensive understanding of this essential chemical concept.
The Electron Transfer: The Heart of Ionic Bonding
The key to understanding ionic bonds lies in the transfer of electrons. Unlike covalent bonds, where electrons are shared between atoms, ionic bonds involve a complete transfer of one or more electrons from one atom to another. This transfer results in the formation of ions: atoms with a net electrical charge.
Electrostatic Attraction: The Driving Force
The transferred electrons leave behind a positively charged ion, known as a cation, and are gained by another atom, forming a negatively charged ion, called an anion. The resulting opposite charges attract each other through powerful electrostatic forces, forming the ionic bond. This electrostatic attraction is the driving force behind the formation and stability of ionic compounds.
Electronegativity: The Electron Tug-of-War
The likelihood of an electron transfer during ionic bond formation depends significantly on the concept of electronegativity. Electronegativity measures an atom's ability to attract electrons towards itself within a chemical bond. Atoms with high electronegativity strongly attract electrons, while those with low electronegativity hold onto their electrons less tightly.
When atoms with significantly different electronegativities interact, the atom with higher electronegativity will pull electrons away from the atom with lower electronegativity. This difference in electronegativity is crucial for the formation of an ionic bond. A large electronegativity difference indicates a greater likelihood of complete electron transfer, leading to the formation of ions and, subsequently, an ionic bond.
Consider the classic example of sodium chloride (NaCl), or common table salt. Sodium (Na) has a low electronegativity, while chlorine (Cl) has a high electronegativity. When they interact, chlorine's strong electronegativity pulls an electron away from sodium. Sodium loses an electron, becoming a positively charged Na⁺ ion (sodium cation), and chlorine gains an electron, becoming a negatively charged Cl⁻ ion (chloride anion). The electrostatic attraction between Na⁺ and Cl⁻ forms the ionic bond in NaCl.
Ionization Energy and Electron Affinity: The Energy Landscape
The process of electron transfer is not spontaneous; it involves energy changes. Two key concepts help understand this energy landscape:
Ionization Energy: The Cost of Losing an Electron
Ionization energy is the energy required to remove an electron from a neutral atom in the gaseous phase. The first ionization energy refers to removing the first electron, the second ionization energy to removing the second, and so on. Generally, ionization energy increases across a period (left to right on the periodic table) and decreases down a group (top to bottom). Atoms with low ionization energy readily lose electrons, making them good candidates to form cations in ionic bonds. Sodium, for example, has a relatively low first ionization energy, readily losing its outermost electron.
Electron Affinity: The Energy Gain from Accepting an Electron
Electron affinity is the energy change that occurs when an atom gains an electron. A high electron affinity indicates that an atom readily accepts an electron, releasing energy in the process. Halogens, such as chlorine, bromine, and iodine, exhibit high electron affinities, making them excellent candidates to form anions in ionic bonds. Chlorine, with its high electron affinity, readily accepts the electron lost by sodium, releasing energy and stabilizing the chloride anion.
Lattice Energy: The Stability of the Ionic Crystal
Once ions are formed, they arrange themselves into a highly ordered three-dimensional structure called a crystal lattice. The energy released when ions come together to form this lattice is called lattice energy. Lattice energy is a measure of the strength of the electrostatic attraction between the ions in the crystal lattice. A higher lattice energy indicates a stronger ionic bond and a more stable ionic compound.
The magnitude of lattice energy depends on several factors:
- Charge of the ions: Higher charges lead to stronger electrostatic attraction and higher lattice energy. For example, the lattice energy of MgO (Mg²⁺ and O²⁻) is significantly higher than that of NaCl (Na⁺ and Cl⁻).
- Size of the ions: Smaller ions result in closer proximity between the oppositely charged ions, leading to stronger attraction and higher lattice energy.
- Arrangement of ions: The specific arrangement of ions in the crystal lattice affects the overall strength of the electrostatic interactions.
The release of lattice energy is a crucial factor in the overall energy change during ionic bond formation. The energy released during lattice formation often outweighs the energy required for ionization, making the overall process energetically favorable.
Beyond the Basics: Factors Influencing Ionic Bond Formation
Several other factors influence the formation and characteristics of ionic bonds:
- Polarization: Even with significant electronegativity differences, some degree of electron sharing might occur. This is particularly true when the cation is small and highly charged and the anion is large and easily polarizable. This leads to some covalent character in the bond, even in primarily ionic compounds.
- Coordination number: This refers to the number of oppositely charged ions surrounding a given ion in the crystal lattice. The coordination number affects the overall stability and packing efficiency of the crystal.
- Solubility: Ionic compounds often dissolve readily in polar solvents, like water, because the polar solvent molecules can interact with the charged ions, overcoming the electrostatic attractions holding the lattice together.
Examples of Ionic Compounds and Their Applications
Ionic compounds are ubiquitous, finding applications in numerous fields. Here are a few examples:
- Sodium Chloride (NaCl): Table salt, essential in our diet and used extensively in various industrial processes.
- Calcium Carbonate (CaCO₃): A major component of limestone, used in construction and as a source of calcium.
- Potassium Chloride (KCl): Used as a fertilizer and in medicine.
- Magnesium Oxide (MgO): Used as a refractory material, in medicine, and as an antacid.
- Silver Chloride (AgCl): Used in photography.
These are just a few examples; countless other ionic compounds have critical roles in various aspects of our lives.
Conclusion: A Deep Dive into Ionic Bonding
Ionic bonding, driven by the transfer of electrons between atoms with significantly different electronegativities, is a fundamental chemical concept. Understanding ionization energy, electron affinity, and lattice energy provides a complete picture of the energetic factors involved in ionic bond formation. The resulting electrostatic attraction between oppositely charged ions leads to the formation of stable ionic compounds, which have diverse applications across various scientific and technological fields. This detailed exploration highlights the intricacies of ionic bonding, providing a solid foundation for further study in chemistry and related disciplines. The transfer of electrons is not merely a simple process; it's a complex interplay of energetic factors that determine the stability and properties of the resulting ionic compounds. By understanding these factors, we can gain a deeper appreciation for the fundamental nature of matter and the world around us.
Latest Posts
Latest Posts
-
How Far Is 1 4 Mile
Mar 18, 2025
-
How Many Phosphate Groups Does Atp Have
Mar 18, 2025
-
Is The Number 31 Prime Or Composite
Mar 18, 2025
-
Highest Common Factor Of 24 And 28
Mar 18, 2025
-
What Is 35 As A Fraction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about When An Ionic Bond Forms Electrons Are . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
