How Many Phosphate Groups Does Atp Have
listenit
Mar 18, 2025 · 6 min read
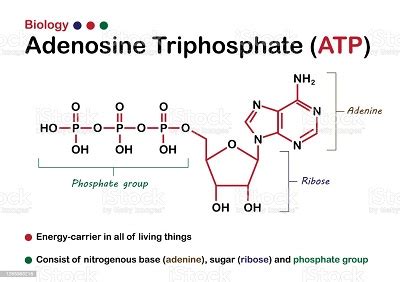
Table of Contents
How Many Phosphate Groups Does ATP Have? Unraveling the Energy Currency of Life
Adenosine triphosphate (ATP) is often called the "energy currency" of life. Its crucial role in powering countless cellular processes makes understanding its structure, particularly the number of phosphate groups it possesses, fundamentally important. This article delves deep into the structure of ATP, explaining why the three phosphate groups are so vital, exploring the energy transfer mechanisms, and touching upon the implications of ATP's unique chemical composition.
The Structure of ATP: A Closer Look
ATP, as its name suggests, is composed of three key components:
- Adenine: A nitrogenous base, a crucial component of DNA and RNA, contributing to ATP's information-carrying potential within the cell.
- Ribose: A five-carbon sugar, forming the backbone of the molecule and providing the structural framework for the attachment of adenine and the phosphate groups.
- Three Phosphate Groups: This is the defining feature of ATP, responsible for its energy storage capacity. These three phosphate groups are linked together via high-energy phosphoanhydride bonds. This is the key answer: ATP has three phosphate groups.
These phosphate groups are typically designated as alpha (α), beta (β), and gamma (γ), starting from the phosphate group closest to the ribose sugar. The bonds connecting these phosphate groups are crucial to ATP's function as an energy carrier.
High-Energy Phosphoanhydride Bonds: The Secret to ATP's Power
The bonds between the phosphate groups in ATP are not ordinary chemical bonds. They are high-energy phosphoanhydride bonds, characterized by a high negative charge density due to the presence of multiple negatively charged oxygen atoms. This repulsion creates significant instability, making these bonds relatively easy to break. When these bonds are hydrolyzed (broken down by the addition of water), a significant amount of energy is released, which can be harnessed by the cell to fuel various biological processes.
The hydrolysis of ATP to adenosine diphosphate (ADP) and inorganic phosphate (Pi) is the most common reaction associated with energy release:
ATP + H₂O → ADP + Pi + Energy
The Role of ATP in Cellular Processes: A Multifaceted Energy Source
The energy released from ATP hydrolysis powers a vast array of cellular activities, including:
- Muscle Contraction: The sliding filament theory relies heavily on ATP to power the myosin motor proteins, facilitating muscle contraction and movement. Without ATP, muscles would be unable to relax or contract, leading to paralysis.
- Active Transport: Moving molecules across cell membranes against their concentration gradients, such as the sodium-potassium pump, requires energy input from ATP. This is essential for maintaining cellular homeostasis and transporting necessary nutrients and ions.
- Protein Synthesis: The intricate process of protein synthesis, from transcription to translation, consumes significant ATP. The energy is required for various steps, including amino acid activation and ribosome function.
- Nerve Impulse Transmission: The transmission of nerve impulses relies on the movement of ions across nerve cell membranes, a process fueled by ATP. This ensures rapid communication throughout the nervous system.
- Cell Signaling: ATP plays a crucial role in various signaling pathways, acting as a signaling molecule itself or influencing other signaling cascades. This is vital for coordinating cellular responses and communication within the organism.
- DNA Replication and Repair: The processes of DNA replication and repair, essential for maintaining genetic integrity, require ATP for enzymes to function correctly. This ensures the accurate passing of genetic information to daughter cells.
- Biosynthesis: The synthesis of numerous biological molecules, such as carbohydrates, lipids, and nucleic acids, relies on ATP's energy input. These processes are crucial for growth, repair, and maintaining cellular structure.
Beyond ATP: Other Energy-Carrying Molecules
While ATP is the primary energy currency, other molecules also contribute to cellular energy transfer. These include:
- Adenosine Diphosphate (ADP): ADP is the product of ATP hydrolysis and can be re-phosphorylated to ATP through various metabolic pathways. This continuous cycle of ATP synthesis and hydrolysis maintains the cellular energy balance.
- Creatine Phosphate: This molecule, particularly abundant in muscle cells, acts as a short-term energy reserve. It can rapidly transfer its phosphate group to ADP, forming ATP when energy demand exceeds immediate ATP supply.
- Guanosine Triphosphate (GTP): GTP is a similar molecule to ATP and plays a role in energy transfer and signaling pathways, especially in protein synthesis.
ATP Synthesis: How the Energy Currency is Generated
The generation of ATP is a crucial process, primarily occurring through two main mechanisms:
- Oxidative Phosphorylation: This occurs in the mitochondria and is the most efficient method of ATP production. It involves the electron transport chain and chemiosmosis, utilizing the energy released from the oxidation of glucose and other fuel molecules to pump protons across the mitochondrial membrane, creating a proton gradient. This gradient drives ATP synthase, an enzyme that generates ATP.
- Substrate-Level Phosphorylation: This is a less efficient but faster method of ATP synthesis, occurring directly during glycolysis and the citric acid cycle. It involves the transfer of a phosphate group from a substrate molecule directly to ADP, forming ATP.
The Significance of the Three Phosphate Groups: A Recap
The presence of three phosphate groups in ATP is not arbitrary; it is fundamentally linked to its function as an energy carrier. The high-energy phosphoanhydride bonds between these groups are responsible for the large amount of energy released upon hydrolysis. This energy is then harnessed by the cell to power a myriad of vital processes, ensuring the survival and function of the organism. The instability caused by the negative charges on the phosphate groups allows for the efficient and controlled release of energy when needed. A change in just one phosphate group dramatically alters the energy and functionality of the molecule.
ATP and Human Health: Implications of Dysfunction
Disruptions in ATP production or utilization can have severe consequences for human health. Mitochondrial diseases, for instance, often involve defects in oxidative phosphorylation, leading to energy deficiency in various tissues and organs. This can manifest in a wide range of symptoms, depending on the affected tissues. Similarly, certain genetic disorders can impair the enzymes involved in ATP synthesis or its utilization.
Furthermore, understanding ATP's role is vital in many areas of medicine and research, including:
- Cancer research: Cancer cells often exhibit altered metabolism and increased ATP demands.
- Neurological disorders: Many neurological diseases are linked to impaired ATP production or utilization in nerve cells.
- Cardiovascular disease: Heart function relies heavily on adequate ATP production.
- Muscle diseases: Muscle disorders can stem from defects in ATP production or utilization.
Conclusion: The Unparalleled Importance of ATP
The simple yet profound structure of ATP, with its three phosphate groups, underpins the very essence of life. The energy released from its hydrolysis drives countless cellular processes, from muscle contraction to DNA replication. Its central role in cellular metabolism makes it a critical target for research in various fields, from medicine to biotechnology. Understanding the intricacies of ATP’s structure and function is crucial for unraveling the complexities of life itself and for developing potential therapies for diseases linked to ATP dysfunction. The three phosphate groups are not merely a structural feature; they are the key to understanding the powerhouse of life.
Latest Posts
Latest Posts
-
What Is The First Element In The Periodic Table
Mar 18, 2025
-
Are Oxidation Numbers The Same As Charges
Mar 18, 2025
-
What Is The Fraction Of 1 25
Mar 18, 2025
-
Nine Is What Percent Of 25
Mar 18, 2025
-
What Is The Lcm Of 9 15
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about How Many Phosphate Groups Does Atp Have . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
