What State Of Matter Has The Most Energy
listenit
Mar 26, 2025 · 6 min read
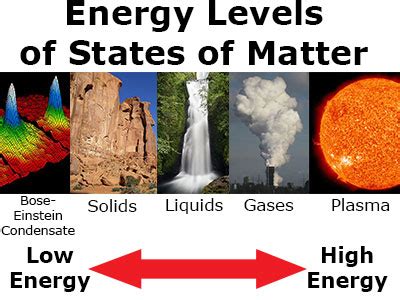
Table of Contents
What State of Matter Has the Most Energy? A Deep Dive into Thermal Energy and Phase Transitions
The question of which state of matter possesses the most energy is deceptively simple. It's not a matter of simply comparing solids, liquids, and gases; the answer hinges on understanding the concept of thermal energy, the specific heat capacity of substances, and the intricacies of phase transitions. While a gas might seem like the obvious answer at first glance, the reality is far more nuanced. This article will explore the complexities of thermal energy within different states of matter, revealing why there isn't a single definitive answer, and highlighting the factors that truly determine the energy content.
Understanding Thermal Energy
Before delving into the specifics of different states of matter, let's establish a foundational understanding of thermal energy. Thermal energy, also known as heat energy, is the total kinetic energy of the particles (atoms and molecules) within a substance. This energy manifests as the movement of these particles – vibration, rotation, and translation. The faster these particles move, the higher the thermal energy and consequently, the higher the temperature.
It's crucial to differentiate between temperature and thermal energy. Temperature is a measure of the average kinetic energy of the particles, while thermal energy represents the total kinetic energy. A large, cold object can possess more thermal energy than a small, hot object because it contains more particles, even if the individual particles have lower average kinetic energies.
Comparing States of Matter: Solids, Liquids, and Gases
The arrangement and movement of particles dictate the properties of each state of matter. This directly impacts their thermal energy content:
Solids: Restricted Movement, Lower Energy?
In solids, particles are tightly packed in a fixed arrangement, exhibiting strong intermolecular forces. Their movement is largely restricted to vibrations around fixed points. This implies relatively lower kinetic energy compared to liquids and gases. However, this doesn't automatically mean solids have the least thermal energy. A large, solid object at a high temperature can contain significantly more thermal energy than a small, hot gas. The total energy depends on both the temperature and the mass of the substance.
Liquids: Increased Mobility, Higher Energy?
Liquids exhibit greater particle mobility than solids. Particles are still relatively close together, but they can move and slide past each other, resulting in greater kinetic energy. The weaker intermolecular forces allow for this increased movement, leading to higher thermal energy compared to solids at the same temperature. However, the density of a liquid generally remains fairly constant, meaning the increase in energy isn't exponentially greater than that of a solid.
Gases: Freedom of Movement, Maximum Kinetic Energy (at a given temperature)?
Gases are characterized by widely dispersed particles with weak intermolecular forces. Particles move freely and randomly, possessing high kinetic energy. At a given temperature, gases typically have a higher thermal energy density compared to liquids and solids due to the much greater freedom of movement. The increased spacing between particles also translates to greater potential energy, further contributing to their overall energy content.
The Role of Phase Transitions
The relationship between thermal energy and the state of matter becomes even more complex when considering phase transitions – the changes between solid, liquid, and gas phases. These transitions involve significant energy changes, even without a change in temperature:
-
Melting (Solid to Liquid): Energy is absorbed to overcome the strong intermolecular forces holding the solid together, allowing particles to move more freely. This energy input increases the thermal energy of the system, even though the temperature remains constant during the phase change.
-
Vaporization (Liquid to Gas): Similarly, energy is absorbed to overcome the intermolecular forces in the liquid, allowing particles to completely escape into the gaseous phase. This requires a significant amount of energy, often much larger than the energy required for melting.
-
Sublimation (Solid to Gas): This direct transition bypasses the liquid phase, requiring a considerable amount of energy to overcome the strong intermolecular forces in the solid and allow particles to enter the gaseous state.
This means that even a small amount of a substance undergoing a phase transition (like water boiling) can absorb a considerable amount of energy, potentially exceeding the thermal energy of a larger mass of a substance in a different phase at a lower temperature.
Beyond the Basics: Plasma and Other States
While solids, liquids, and gases are the most commonly encountered states of matter, there are others, most notably plasma. Plasma is an ionized gas, meaning it consists of charged particles (ions and electrons). The presence of these charged particles introduces new complexities in energy considerations. The kinetic energy of these particles is significantly higher than in a neutral gas at the same temperature, resulting in much higher thermal energy. The strong electromagnetic interactions between the charged particles also contribute to the overall energy content.
Beyond plasma, exotic states of matter exist, such as Bose-Einstein condensates and superfluids, which exhibit unique quantum properties and correspondingly unique energy characteristics.
So, which state of matter has the most energy? The nuanced answer.
There's no single answer to the question of which state of matter has the most energy. The thermal energy content depends critically on:
- Mass: A larger mass of any substance will possess more thermal energy at the same temperature.
- Temperature: Higher temperatures directly translate to higher thermal energy.
- Specific heat capacity: This property reflects how much energy is required to raise the temperature of a substance by a certain amount. Different substances have different specific heat capacities.
- Phase: Phase transitions involve significant energy changes, even without a change in temperature. A small amount of a substance undergoing vaporization might contain far more thermal energy than a large amount of another substance in a different phase at a lower temperature.
- State of matter: At a given temperature, gases generally possess higher thermal energy than liquids, which in turn have higher energy than solids. However, plasma drastically increases the energy density.
Therefore, a large, hot plasma would generally possess the highest thermal energy, whereas a small, cold solid would typically have the least. However, comparing a large, cold solid to a smaller amount of boiling water shows the complexity, as the water undergoing a phase transition might well have more energy.
Understanding the interplay between these factors is crucial to accurately assess the thermal energy content of any substance, regardless of its state of matter. The question isn't just about the inherent properties of solids, liquids, and gases, but also about the macroscopic properties of mass, temperature, and the transformative energy of phase transitions.
Latest Posts
Latest Posts
-
Rna Differs From Dna In That Rna
Mar 29, 2025
-
Why Did The Small States Object To The Virginia Plan
Mar 29, 2025
-
The Force That Holds Two Atoms Together
Mar 29, 2025
-
Is Sulfur A Metal Metalloid Or Nonmetal
Mar 29, 2025
-
How Many Quarts In Five Gallons
Mar 29, 2025
Related Post
Thank you for visiting our website which covers about What State Of Matter Has The Most Energy . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
