The Force That Holds Two Atoms Together
listenit
Mar 29, 2025 · 6 min read
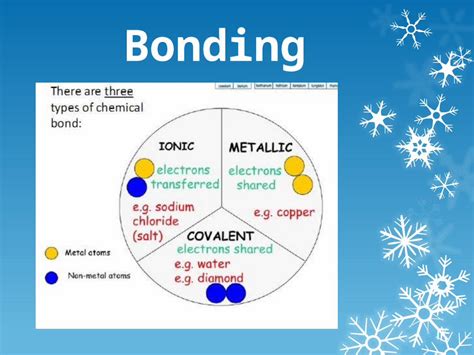
Table of Contents
The Force That Holds Two Atoms Together: A Deep Dive into Chemical Bonding
The seemingly simple question, "What holds two atoms together?" unveils a fascinating world of physics and chemistry. The answer lies in the intricate dance of electrons and the powerful forces governing their interactions: chemical bonding. This article explores the fundamental forces behind chemical bonds, delving into the various types of bonds, their strengths, and the properties they impart to molecules and materials.
Understanding Atomic Structure: The Foundation of Bonding
Before we delve into the forces holding atoms together, let's revisit the basic structure of an atom. An atom consists of a dense, positively charged nucleus containing protons and neutrons, surrounded by a cloud of negatively charged electrons. These electrons occupy specific energy levels or orbitals, dictated by quantum mechanics. The outermost electrons, known as valence electrons, play a crucial role in chemical bonding. Their arrangement dictates an atom's reactivity and the types of bonds it can form.
The Role of Valence Electrons
Valence electrons are the key players in chemical bonding. Atoms tend to interact in ways that achieve a more stable electron configuration. This stability is often associated with a filled valence shell, mimicking the electron configuration of noble gases, which are exceptionally unreactive. Atoms achieve this stability by either gaining, losing, or sharing valence electrons. This drive for stability is the fundamental driving force behind chemical bonding.
Types of Chemical Bonds: A Spectrum of Interactions
Several types of chemical bonds exist, each with unique characteristics and strengths:
1. Ionic Bonds: The Electrostatic Attraction
Ionic bonds arise from the electrostatic attraction between oppositely charged ions. This occurs when one atom readily loses electrons (becoming a positively charged cation) and another atom readily gains electrons (becoming a negatively charged anion). The resulting electrostatic force holds the ions together. This type of bond is characteristic of compounds formed between metals (which tend to lose electrons) and nonmetals (which tend to gain electrons).
Example: Sodium chloride (NaCl), common table salt, is a classic example. Sodium (Na) readily loses one electron to achieve a stable electron configuration, becoming a Na⁺ cation. Chlorine (Cl) readily gains one electron to achieve a stable electron configuration, becoming a Cl⁻ anion. The strong electrostatic attraction between Na⁺ and Cl⁻ ions forms the ionic bond in NaCl.
Key Characteristics of Ionic Bonds:
- High melting and boiling points: The strong electrostatic forces require significant energy to overcome.
- Crystalline structure: Ions arrange themselves in a regular, repeating pattern in a crystal lattice.
- Conductivity in molten or aqueous state: Free-moving ions can carry electric charge.
- Brittle nature: Shifting the crystal lattice can cause like-charged ions to repel, leading to fracture.
2. Covalent Bonds: Sharing is Caring
Covalent bonds involve the sharing of one or more pairs of electrons between atoms. This sharing allows each atom to achieve a more stable electron configuration. Covalent bonds are typically formed between nonmetal atoms, which have similar electronegativities (a measure of an atom's ability to attract electrons).
Example: The simplest example is the hydrogen molecule (H₂). Each hydrogen atom has one valence electron. By sharing their electrons, each hydrogen atom effectively achieves a filled valence shell (like helium). The shared electron pair is attracted to both nuclei, creating a strong bond.
Key Characteristics of Covalent Bonds:
- Lower melting and boiling points than ionic compounds (generally): The forces holding covalent molecules together are generally weaker than the electrostatic forces in ionic compounds.
- Molecular structure: Atoms are linked together in discrete molecules.
- Can be polar or nonpolar: Depending on the electronegativity difference between the bonded atoms.
- Poor conductivity in solid or liquid states (generally): Electrons are localized in the bonds and not free to move.
3. Metallic Bonds: A Sea of Electrons
Metallic bonds are found in metals and alloys. In this type of bond, valence electrons are delocalized; they are not associated with any particular atom but rather move freely throughout the metal lattice. This "sea" of delocalized electrons holds the positively charged metal ions together.
Key Characteristics of Metallic Bonds:
- High electrical and thermal conductivity: The free-moving electrons can readily conduct electricity and heat.
- Malleability and ductility: The delocalized electrons allow the metal ions to slide past each other without breaking the metallic bond.
- Lustrous appearance: The interaction of light with the delocalized electrons results in a shiny appearance.
- Variable melting and boiling points: Dependent on the strength of the metallic bond, which is affected by factors like the number of valence electrons and the size of the metal ions.
4. Hydrogen Bonds: A Special Type of Intermolecular Force
Hydrogen bonds are a special type of intermolecular force (a force between molecules, rather than within a molecule). They occur when a hydrogen atom bonded to a highly electronegative atom (such as oxygen, nitrogen, or fluorine) is attracted to another electronegative atom in a nearby molecule. While weaker than ionic or covalent bonds, hydrogen bonds are crucial in many biological systems and significantly influence the properties of water.
Key Characteristics of Hydrogen Bonds:
- Relatively weak compared to covalent and ionic bonds: Easily broken and reformed.
- Responsible for many unique properties of water: High boiling point, surface tension, and solvent properties.
- Crucial role in biological molecules: Stabilizing the structure of proteins and DNA.
Factors Influencing Bond Strength
The strength of a chemical bond depends on several factors:
- Electronegativity difference: A large difference in electronegativity leads to stronger ionic bonds.
- Number of shared electron pairs: More shared pairs result in stronger covalent bonds.
- Bond length: Shorter bond lengths generally indicate stronger bonds.
- Atomic size: Smaller atoms generally form stronger bonds.
The Importance of Chemical Bonding
Understanding chemical bonding is fundamental to comprehending the properties of matter. The type of bond formed between atoms determines the physical and chemical properties of a substance, including its melting point, boiling point, conductivity, solubility, and reactivity. This knowledge is crucial in various fields, including:
- Materials science: Designing new materials with specific properties.
- Chemistry: Understanding chemical reactions and synthesizing new compounds.
- Biology: Understanding the structure and function of biological molecules.
- Medicine: Developing new drugs and treatments.
Conclusion: A Dynamic and Essential Force
The forces that hold two atoms together are far from static; they are dynamic and complex, governed by the fundamental principles of quantum mechanics and electrostatics. The diverse types of chemical bonds, from the strong electrostatic attraction in ionic bonds to the sharing of electrons in covalent bonds and the unique properties of metallic and hydrogen bonds, dictate the properties of the world around us. A deeper understanding of these forces is crucial for advancing our knowledge and technological capabilities across various scientific disciplines. The seemingly simple question, "What holds two atoms together?" has led us on a journey into the heart of matter, revealing a world of remarkable complexity and elegance.
Latest Posts
Latest Posts
-
Gcf Of 42 126 And 210
Apr 01, 2025
-
What Are The First 5 Multiples Of 7
Apr 01, 2025
-
What Is 8 10 As A Decimal
Apr 01, 2025
Related Post
Thank you for visiting our website which covers about The Force That Holds Two Atoms Together . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
