What Part Of The Atom Is Involved In Chemical Reactions
listenit
Mar 21, 2025 · 6 min read
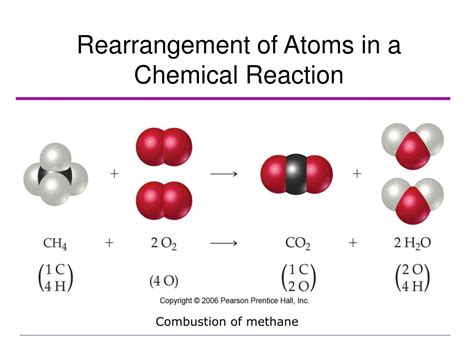
Table of Contents
What Part of the Atom is Involved in Chemical Reactions?
Understanding the intricacies of chemical reactions requires delving into the fundamental building blocks of matter: atoms. While atoms themselves are incredibly small, their internal structure dictates their reactivity and how they interact to form molecules and compounds. This article will explore the specific part of the atom responsible for chemical reactions, examining the roles of protons, neutrons, and, most importantly, electrons. We will also investigate how electron configuration, energy levels, and valence electrons contribute to the diverse chemical behaviors we observe in the world around us.
The Atomic Structure: A Quick Refresher
Before diving into the specifics of chemical reactions, it's crucial to revisit the basic structure of an atom. Atoms consist of three subatomic particles:
- Protons: Positively charged particles located in the atom's nucleus. The number of protons defines the element's atomic number and determines its identity.
- Neutrons: Neutrally charged particles also residing in the nucleus. They contribute to the atom's mass but not its charge. Different isotopes of an element have varying numbers of neutrons.
- Electrons: Negatively charged particles orbiting the nucleus in specific energy levels or shells. These electrons are far less massive than protons and neutrons but play a crucial role in chemical bonding and reactivity.
The Key Player: Electrons and Their Role in Chemical Reactions
While protons and neutrons contribute to an atom's identity and mass, they are largely uninvolved in chemical reactions. The reason for this lies in their location and the strong nuclear forces that bind them together within the atom's nucleus. These forces are far too strong to be easily overcome during typical chemical interactions.
In contrast, electrons, residing in the outermost shell (valence shell), are the primary participants in chemical reactions. These valence electrons are loosely held by the atom and can be shared, gained, or lost during interactions with other atoms. This transfer or sharing of electrons forms the basis of chemical bonds, which hold atoms together to create molecules and compounds.
Valence Electrons: The Architects of Chemical Bonding
The number of valence electrons an atom possesses dictates its chemical behavior and the types of bonds it can form. Atoms strive for stability, which is often achieved by having a full outermost electron shell. This principle is often referred to as the octet rule, where atoms tend to gain, lose, or share electrons to achieve a stable configuration of eight valence electrons (except for hydrogen and helium, which require only two electrons for stability).
Different types of chemical bonds arise from the interactions of valence electrons:
-
Ionic Bonds: Formed through the complete transfer of electrons from one atom to another. This results in the formation of ions – charged atoms – with one atom becoming positively charged (cation) and the other becoming negatively charged (anion). The electrostatic attraction between these oppositely charged ions creates the ionic bond. For example, the formation of sodium chloride (NaCl) involves the transfer of an electron from sodium (Na) to chlorine (Cl).
-
Covalent Bonds: Formed when atoms share one or more pairs of electrons. This type of bond is common between nonmetal atoms, which tend to have high electronegativities. Covalent bonds can be polar (unequal sharing of electrons) or nonpolar (equal sharing of electrons), depending on the electronegativity difference between the atoms involved. For example, the formation of water (H₂O) involves covalent bonds between oxygen and hydrogen atoms.
-
Metallic Bonds: Found in metals, where valence electrons are delocalized and move freely throughout the metal lattice. This "sea" of electrons allows for the high electrical and thermal conductivity characteristic of metals.
Electron Configuration and Energy Levels: A Deeper Dive
The arrangement of electrons in an atom's different energy levels is crucial in understanding chemical reactivity. Electrons occupy specific energy levels or shells, with each level capable of holding a limited number of electrons. The further an electron is from the nucleus, the higher its energy level and the more likely it is to participate in a chemical reaction.
The electron configuration of an atom, which describes the arrangement of electrons in its energy levels, is usually represented using spectroscopic notation. This notation shows the number of electrons in each subshell (s, p, d, f). For instance, the electron configuration of oxygen is 1s²2s²2p⁴, indicating two electrons in the 1s subshell, two in the 2s subshell, and four in the 2p subshell. The valence electrons are those in the highest energy level (in this case, the 2p subshell).
The distribution of electrons among energy levels and subshells also influences the atom's electronegativity – its tendency to attract electrons towards itself in a chemical bond. Atoms with high electronegativity tend to attract electrons more strongly, making them more likely to form ionic or polar covalent bonds.
Beyond Valence Electrons: Other Factors Affecting Reactivity
While valence electrons are the primary players in chemical reactions, other factors also influence an atom's reactivity:
- Atomic Size: Larger atoms generally have lower electronegativity and are less likely to attract electrons, making them less reactive.
- Ionization Energy: The energy required to remove an electron from an atom. Atoms with low ionization energy readily lose electrons and are more reactive.
- Electron Affinity: The energy change when an atom gains an electron. Atoms with high electron affinity readily gain electrons and are more reactive.
- Shielding Effect: Inner electrons shield outer electrons from the full positive charge of the nucleus, affecting the effective nuclear charge experienced by valence electrons. This shielding effect influences both electronegativity and reactivity.
Examples of Chemical Reactions and the Role of Electrons
Let's consider a few examples to solidify the understanding of how electrons dictate chemical reactivity:
1. Formation of Sodium Chloride (NaCl): Sodium (Na) has one valence electron, while chlorine (Cl) has seven. Sodium readily loses its valence electron to achieve a stable configuration, becoming a positively charged Na⁺ ion. Chlorine readily gains this electron, achieving a stable configuration and becoming a negatively charged Cl⁻ ion. The electrostatic attraction between Na⁺ and Cl⁻ ions forms the ionic bond in NaCl.
2. Formation of Water (H₂O): Oxygen (O) has six valence electrons and needs two more to achieve a stable configuration. Each hydrogen (H) atom has one valence electron. Oxygen shares one electron with each hydrogen atom, forming two covalent bonds. This sharing of electrons results in the formation of a stable water molecule.
3. Combustion of Methane (CH₄): Methane (CH₄) reacts with oxygen (O₂) in a combustion reaction to produce carbon dioxide (CO₂) and water (H₂O). This reaction involves the breaking and forming of covalent bonds. The electrons in the methane molecule are rearranged during the reaction, forming new bonds with oxygen atoms.
Conclusion: The Electron's Central Role
In conclusion, electrons, particularly valence electrons, are the primary subatomic particles involved in chemical reactions. Their ability to be shared, gained, or lost determines the type of chemical bonds formed and the overall reactivity of atoms. Understanding the electron configuration, energy levels, and other factors influencing electron behavior is essential for comprehending the vast array of chemical reactions that shape our world, from the formation of simple molecules to complex biological processes. While the nucleus plays a role in determining the properties of an element, it's the electron's dance around the nucleus that dictates the vibrant and ever-changing world of chemistry.
Latest Posts
Latest Posts
-
How To Multiply 2x2 Matrix By 2x1
Mar 28, 2025
-
Square Root Of 3 Root 5
Mar 28, 2025
-
Integral Of X Sqrt X 1
Mar 28, 2025
-
3 Main Ideas Of Cell Theory
Mar 28, 2025
-
What Are The Most Reactive Metals In The Periodic Table
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Part Of The Atom Is Involved In Chemical Reactions . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
