What Is The Trend Of Atomic Radii
listenit
Mar 27, 2025 · 6 min read
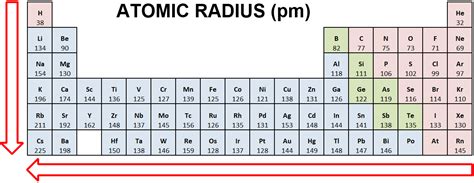
Table of Contents
What is the Trend of Atomic Radii? A Comprehensive Exploration
Atomic radius, a fundamental concept in chemistry, describes the size of an atom. Understanding its trends across the periodic table is crucial for predicting chemical properties and behaviors. This comprehensive exploration delves into the intricacies of atomic radius, explaining its trends, the factors influencing them, and their implications in various chemical phenomena.
Defining Atomic Radius: More Than Just a Simple Measurement
Defining atomic radius isn't straightforward. Atoms don't have sharply defined boundaries like billiard balls. Instead, the electron cloud surrounding the nucleus gradually fades away. Therefore, several methods exist to determine atomic size, each yielding slightly different values. These methods include:
1. Metallic Radius:
This refers to half the distance between two adjacent nuclei in a metallic crystal lattice. It's applicable to metals, where atoms are tightly packed.
2. Covalent Radius:
This is half the distance between the nuclei of two identical atoms bonded covalently. It's used for non-metal atoms.
3. Van der Waals Radius:
This represents half the distance between the nuclei of two identical non-bonded atoms. It's the largest of the three, reflecting the weaker interactions between non-bonded atoms.
While the specific values may vary depending on the method, the overall trends remain consistent across the periodic table.
The Periodic Trend of Atomic Radius: Across and Down
The atomic radius exhibits clear trends across periods (rows) and down groups (columns) of the periodic table:
1. Across a Period (Left to Right):
Atomic radius generally decreases across a period. This is primarily due to the increasing effective nuclear charge. As you move from left to right, the number of protons in the nucleus increases, while the number of shielding electrons in the same principal energy level remains relatively constant. This stronger positive charge pulls the electrons closer to the nucleus, resulting in a smaller atomic radius.
Stronger Nuclear Pull: The increased nuclear charge exerts a stronger attractive force on the electrons, overcoming the slight increase in electron-electron repulsion.
Shielding Effect: While inner electrons shield the outer electrons from the full nuclear charge, this shielding effect is not sufficient to counteract the increasing nuclear charge across a period.
Example: Comparing lithium (Li) to fluorine (F) in the second period, fluorine has significantly smaller atomic radius because of the increased nuclear charge and similar shielding effect.
2. Down a Group (Top to Bottom):
Atomic radius generally increases down a group. This is because new electron shells are added as you move down a group. Each new shell resides farther from the nucleus, increasing the overall size of the atom.
Increased Principal Quantum Number: The addition of electron shells leads to an increase in the principal quantum number (n), representing the energy level and distance from the nucleus.
Increased Shielding: The increased number of electron shells enhances the shielding effect, reducing the effective nuclear charge felt by the outermost electrons.
Example: Comparing lithium (Li) to sodium (Na) in group 1, sodium has a significantly larger atomic radius because of the addition of a new electron shell.
Factors Influencing Atomic Radius: A Deeper Dive
Several factors interplay to determine the size of an atom:
1. Effective Nuclear Charge (Zeff):
Zeff represents the net positive charge experienced by an electron in an atom. It's the difference between the actual nuclear charge and the shielding effect of inner electrons. A higher Zeff leads to a stronger pull on the outer electrons, resulting in a smaller atomic radius.
2. Shielding Effect:
Inner electrons shield outer electrons from the full nuclear charge. The more inner electrons present, the less effective the nuclear charge is on the outer electrons, resulting in a larger atomic radius. This shielding effect is less effective for electrons in the same principal energy level.
3. Number of Electron Shells:
Adding a new electron shell significantly increases the atomic radius. Each shell corresponds to a higher principal quantum number and thus a greater average distance from the nucleus.
4. Electron-Electron Repulsion:
The repulsion between electrons in the same shell slightly increases the atomic radius. However, this effect is usually weaker than the effect of effective nuclear charge.
Exceptions and Anomalies: Understanding Deviations from the Trend
While the general trends are clear, certain exceptions and anomalies can be observed. These are often attributed to specific electron configurations or inter-electron interactions:
1. Transition Metals:
Across a transition metal series, the atomic radius changes less drastically than in other periods. This is because the added electrons are filling inner d orbitals, which provide less shielding to the outer s electrons. The increased nuclear charge partially offsets the shielding effect.
2. Lanthanides and Actinides:
The lanthanide and actinide series show a unique trend of decreasing atomic radius as you move across the series (lanthanide contraction). This is due to the poor shielding effect of the f electrons, resulting in a greater effective nuclear charge and smaller atomic radius.
3. Anomalous Pairs:
Certain pairs of elements, due to electron configuration differences, might deviate from the expected trend. These variations are often subtle and require a deeper analysis of electron-electron interactions and orbital shapes.
Implications of Atomic Radius Trends: Real-World Applications
Understanding the trends in atomic radius is crucial in several areas of chemistry and related fields:
1. Predicting Chemical Reactivity:
Atomic radius influences an atom's ability to gain or lose electrons, thus impacting its reactivity. Smaller atoms with high effective nuclear charges tend to be more electronegative, while larger atoms with lower effective nuclear charges tend to be more electropositive.
2. Ionic Radius and Crystal Structure:
The formation of ions involves changes in electron number and consequently atomic radius. The size of ions plays a significant role in determining the structure and properties of ionic compounds, such as lattice energy and solubility.
3. Metallic Bonding and Properties:
The atomic radius influences the strength of metallic bonding and the properties of metals, such as conductivity and ductility.
4. Covalent Bonding and Molecular Shape:
The size of atoms involved in covalent bonding affects bond length and molecular geometry, impacting physical and chemical properties.
Conclusion: Atomic Radius – A Cornerstone of Chemical Understanding
Atomic radius, while seemingly a simple concept, is a cornerstone of our understanding of chemical behavior. The consistent trends across the periodic table, coupled with a deep understanding of the underlying factors, allow us to predict and explain a wide range of chemical phenomena. From predicting reactivity to understanding crystal structures, grasping the nuances of atomic radius is essential for anyone delving into the fascinating world of chemistry. The exceptions and anomalies only highlight the complexity and beauty of the periodic system, reminding us that seemingly simple trends often hide intricate interactions at the atomic level. The continued study and exploration of atomic radii will undoubtedly lead to further advancements in our understanding of chemical principles and applications.
Latest Posts
Latest Posts
-
Least Common Multiple Of 4 And 3
Mar 30, 2025
-
How Many Lines Of Symmetry Does A Regular Decagon Have
Mar 30, 2025
-
How Does An Igneous Rock Become A Sedimentary Rock
Mar 30, 2025
-
The Mass Of One Mole Of Carbon Dioxide Is
Mar 30, 2025
-
20 Is 85 Of What Number
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Trend Of Atomic Radii . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
