What Is The Monomer Of A Lipid
listenit
Mar 18, 2025 · 6 min read
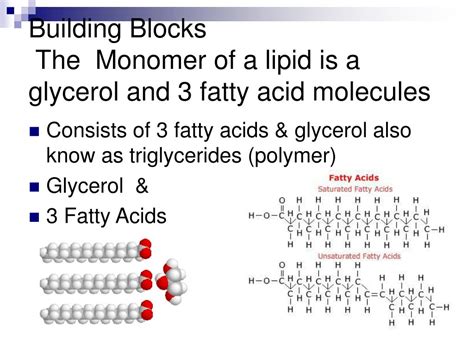
Table of Contents
What is the Monomer of a Lipid? Understanding the Building Blocks of Fats and Oils
Lipids, a diverse group of biological molecules, are often characterized by their insolubility in water. While we commonly think of fats and oils, lipids encompass a much broader range of molecules with vital roles in cell structure, energy storage, and signaling. Unlike carbohydrates and proteins that have clear monomeric units (monosaccharides and amino acids, respectively), the question of a singular "monomer" for lipids is more nuanced. This is because different types of lipids are built from different building blocks. Instead of a single monomer, we should focus on the fundamental components that make up various lipid classes. This article will delve into the diverse world of lipids, exploring their structures and the components that constitute their unique properties.
The Misconception of a Single Lipid Monomer
It's important to dispel the notion that lipids possess one single monomer like proteins (amino acids) or carbohydrates (monosaccharides). The term "monomer" implies a repeating unit that polymerizes to form a larger macromolecule. While some lipids, like triglycerides, are assembled from smaller subunits, the process isn't strictly polymerization in the same way as protein or carbohydrate synthesis. The different classes of lipids have distinct building blocks:
-
Fatty Acids: These are long hydrocarbon chains with a carboxyl group at one end. They are crucial components of many lipids, serving as the building blocks for triglycerides, phospholipids, and sphingolipids. Fatty acids themselves aren't monomers in the classical sense, but they are the fundamental components used to build more complex lipid structures.
-
Glycerol: This is a three-carbon alcohol with three hydroxyl (-OH) groups. It acts as a backbone for triglycerides and phospholipids, linking fatty acids together. While not a monomer in the strictest definition, it plays a pivotal role in lipid assembly.
-
Phosphate: A key component of phospholipids, the phosphate group contributes to the hydrophilic (water-loving) head of the phospholipid molecule, contributing to the formation of cell membranes.
-
Sphingosine: This is a long-chain amino alcohol that forms the backbone of sphingolipids, a class of lipids crucial for cell signaling and membrane structure. Like glycerol, it's not a monomer in the traditional sense, but a crucial structural component.
-
Steroid Nucleus: Steroids, like cholesterol, are characterized by their four fused carbon rings. They are synthesized from isoprene units, which are five-carbon molecules. Although isoprene units are considered precursors, they aren't precisely the "monomers" of steroids in the same way as amino acids are for proteins.
Exploring Key Lipid Classes and Their Components
Let's examine the main lipid classes to better understand their constituent parts:
1. Triglycerides (Triglycerides): The Energy Storage Champions
Triglycerides are the most common type of lipid, primarily functioning as energy storage molecules. They are composed of:
- One glycerol molecule: Providing the backbone structure.
- Three fatty acid molecules: Ester-linked to each of the glycerol's hydroxyl groups. These fatty acids can be saturated (no double bonds), monounsaturated (one double bond), or polyunsaturated (multiple double bonds), influencing the triglyceride's physical properties (e.g., solid fat vs. liquid oil).
The esterification of glycerol with three fatty acids is a condensation reaction, not a polymerization reaction. This is a crucial distinction when discussing lipid monomers. The fatty acids themselves are not repeating units but rather individual components contributing to the triglyceride structure.
2. Phospholipids: The Membrane Architects
Phospholipids are the primary building blocks of cell membranes. Their amphipathic nature—possessing both hydrophilic and hydrophobic regions—is crucial for membrane formation. They typically consist of:
- One glycerol molecule: Forms the backbone.
- Two fatty acid molecules: Ester-linked to two of glycerol's hydroxyl groups.
- One phosphate group: Esterified to the third hydroxyl group of glycerol.
- A polar head group: Attached to the phosphate group. This head group can vary, influencing the phospholipid's properties. Common head groups include choline, serine, ethanolamine, and inositol.
The assembly of a phospholipid is again a condensation reaction, not polymerization. While there are repeating units of fatty acids and the polar head, these are not monomers in the traditional sense used for polymers.
3. Sphingolipids: Signaling and Membrane Stability
Sphingolipids are complex lipids found in cell membranes, particularly abundant in the nervous system. They are constructed using:
- One sphingosine molecule: A long-chain amino alcohol that forms the backbone.
- One fatty acid molecule: Attached via an amide linkage to the sphingosine amino group.
- A polar head group: Attached to the sphingosine hydroxyl group. This can be a variety of molecules, including carbohydrates (glycosphingolipids) or phosphocholine (sphingomyelins).
The structure of sphingolipids emphasizes that the concept of a single monomer for all lipids is unrealistic. The components are assembled through specific reactions, not the polymerization seen in other biomolecules.
4. Steroids: Multifaceted Roles in Biology
Steroids, like cholesterol and steroid hormones, are characterized by their four fused carbon rings. They are synthesized from:
- Isoprene units: These five-carbon molecules are the precursors to the steroid nucleus. The process of steroid biosynthesis involves multiple enzymatic steps, not simple polymerization. Therefore, isoprene is more of a precursor than a strict monomer.
Cholesterol, a crucial component of animal cell membranes, doesn't have a repeating monomeric unit. Its unique structure and function distinguish it from other lipid classes.
The Importance of Understanding Lipid Structure
Understanding the components of different lipid classes is crucial for appreciating their diverse functions. The variations in fatty acid chains, polar head groups, and backbone structures determine the lipid's properties:
- Hydrophobicity/Hydrophilicity: Affects membrane permeability and interactions with water.
- Melting Point: Influences the physical state of the lipid (solid or liquid) at different temperatures.
- Membrane Fluidity: Impacts the cell membrane's flexibility and stability.
- Cell Signaling: Sphingolipids and other lipids play critical roles in cell communication.
- Energy Storage: Triglycerides are crucial for long-term energy storage.
Conclusion: Beyond the Monomer Concept
While the term "monomer" is often used to describe the building blocks of polymers like proteins and carbohydrates, it's less applicable to the diverse world of lipids. Different lipid classes have distinct structural components, and these components are assembled through various reactions, not polymerization. Instead of searching for a single lipid monomer, it's more accurate to focus on the key building blocks: fatty acids, glycerol, sphingosine, phosphate, and isoprene units. Understanding the specific composition and arrangement of these components is crucial for comprehending the structure, function, and biological significance of different lipids. The intricate interplay of these building blocks contributes to the remarkable diversity and essential roles of lipids in living organisms. Further research into lipid biosynthesis and function continues to reveal new insights into their importance in health and disease.
Latest Posts
Latest Posts
-
What Make Up The Rungs Of Dna
Mar 18, 2025
-
Of The Employees Who Work At Stalling Printing
Mar 18, 2025
-
Where Is The Dna In A Eukaryotic Cell Located
Mar 18, 2025
-
What Is Cl In The Periodic Table
Mar 18, 2025
-
Molecules Are Formed By The Bonding Together Of
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is The Monomer Of A Lipid . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
