What Is Cl In The Periodic Table
listenit
Mar 18, 2025 · 6 min read
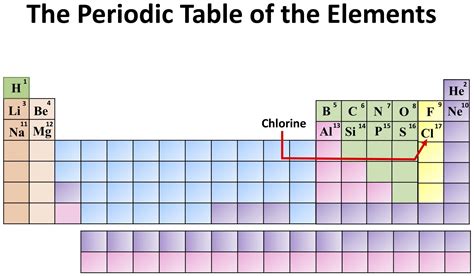
Table of Contents
What is Cl in the Periodic Table? A Deep Dive into Chlorine
Chlorine (Cl), element number 17 on the periodic table, is a fascinating and vital element. While its name might conjure images of swimming pools and household cleaning products, its role extends far beyond these common applications. This comprehensive article delves into the properties, reactions, uses, and environmental impact of chlorine, providing a complete understanding of this multifaceted halogen.
Understanding Chlorine's Position in the Periodic Table
Chlorine resides in Group 17, also known as the halogens, and Period 3 of the periodic table. This placement dictates many of its key characteristics.
Group 17: The Halogens
The halogens are a family of highly reactive nonmetals. They all have seven valence electrons, meaning they need only one more electron to achieve a stable octet configuration. This strong desire for an extra electron drives their highly reactive nature. Other halogens include fluorine (F), bromine (Br), iodine (I), and astatine (At). As you move down the group, reactivity generally decreases.
Period 3: Atomic Structure and Properties
Chlorine's position in Period 3 indicates it has three electron shells. Its electronic configuration is 1s²2s²2p⁶3s²3p⁵, highlighting the seven valence electrons in the outermost shell. This configuration explains its strong electronegativity – its tendency to attract electrons towards itself in a chemical bond.
Physical and Chemical Properties of Chlorine
Chlorine's properties are directly linked to its atomic structure and position on the periodic table.
Physical Properties
- State at Room Temperature: Chlorine exists as a yellowish-green gas at room temperature and standard pressure. This is a distinguishing characteristic of the halogens.
- Odor: It has a pungent, suffocating odor, easily detectable even in low concentrations. This characteristic odor is often used as a warning sign of its presence.
- Solubility: Chlorine is moderately soluble in water, forming hypochlorous acid (HOCl) and hydrochloric acid (HCl), contributing to its disinfecting properties.
- Boiling Point and Melting Point: Chlorine has a relatively low boiling point (-34.04 °C) and melting point (-101.5 °C) compared to other elements, reflecting its relatively weak intermolecular forces.
- Density: Chlorine gas is denser than air.
Chemical Properties
- High Reactivity: As a halogen, chlorine is highly reactive, readily forming compounds with many other elements. Its strong electronegativity makes it an excellent oxidizing agent.
- Oxidation States: Chlorine can exhibit various oxidation states, ranging from -1 (chloride ion, Cl⁻) to +7 (perchlorate ion, ClO₄⁻). This versatility allows it to participate in a wide range of chemical reactions.
- Formation of Ionic Compounds: Chlorine readily forms ionic compounds with metals, accepting an electron to achieve a stable octet and forming chloride ions (Cl⁻). Examples include sodium chloride (NaCl), commonly known as table salt, and calcium chloride (CaCl₂).
- Formation of Covalent Compounds: Chlorine also forms covalent compounds by sharing electrons with other nonmetals. Examples include hydrogen chloride (HCl), a strong acid, and carbon tetrachloride (CCl₄), a previously widely used solvent.
- Disproportionation: Chlorine can undergo disproportionation, where it simultaneously acts as both an oxidizing and reducing agent. This is evident in its reaction with water, forming both hypochlorous acid (HOCl) and hydrochloric acid (HCl).
Production and Uses of Chlorine
Chlorine's versatile chemical properties make it an indispensable element in numerous industries.
Industrial Production
The primary method of industrial chlorine production is the electrolysis of brine (sodium chloride solution). This process separates sodium chloride into its constituent ions, sodium (Na⁺) and chloride (Cl⁻). The chloride ions are then oxidized at the anode, producing chlorine gas. This process simultaneously produces sodium hydroxide (NaOH) and hydrogen gas (H₂), making it an economically important process.
Major Uses
- Water Treatment: Chlorine is a widely used disinfectant in water treatment plants, effectively killing harmful bacteria and viruses, making it safe for consumption. Its effectiveness and relatively low cost make it a mainstay of water purification.
- Bleach Production: Chlorine is a key component in the production of sodium hypochlorite (NaClO), the active ingredient in many household bleaches. Its bleaching action is due to its oxidizing properties, which break down colored compounds.
- PVC Production: Polyvinyl chloride (PVC), a versatile plastic used in pipes, flooring, and many other applications, is produced using chlorine. The chlorine atoms are crucial for the polymer's stability and properties.
- Solvent Production: Chlorinated solvents, such as carbon tetrachloride (CCl₄), were previously used extensively, although their use has declined due to environmental concerns.
- Pharmaceuticals and Pesticides: Chlorine is a component in the synthesis of many pharmaceuticals and pesticides. However, the use of chlorine-containing pesticides is decreasing due to environmental concerns.
- Other Industrial Applications: Chlorine finds use in various other industrial processes, including the production of dyes, textiles, and other chemicals.
Environmental Impact and Safety Concerns
Despite its numerous benefits, chlorine presents some environmental and safety challenges.
Environmental Concerns
- Ozone Depletion: Certain chlorofluorocarbons (CFCs), which contain chlorine, were once widely used as refrigerants and propellants. However, their release into the atmosphere contributed to ozone depletion, leading to the Montreal Protocol, an international treaty phasing out their production and use.
- Water Pollution: Discharge of chlorine-containing waste into water bodies can harm aquatic life. Proper treatment and disposal of chlorine-containing waste are crucial for environmental protection.
- Dioxins and Furans: The incineration of chlorine-containing materials can lead to the formation of dioxins and furans, highly toxic pollutants. Proper waste management is essential to minimize their formation.
Safety Concerns
- Toxicity: Chlorine gas is toxic and can cause respiratory irritation, even at low concentrations. Exposure to high concentrations can be fatal. Proper safety precautions, including respiratory protection, are essential when handling chlorine.
- Reactivity: Chlorine's high reactivity requires careful handling and storage to prevent accidental reactions and fires.
- First Aid: In case of chlorine exposure, immediate removal from the contaminated area and fresh air are crucial. Medical attention should be sought promptly.
Chlorine Compounds: A Diverse Range
The versatility of chlorine extends to the wide range of compounds it forms.
Chlorides
Metal chlorides, like sodium chloride (NaCl), are common ionic compounds with widespread applications, from table salt to de-icing agents. These compounds are typically soluble in water, dissociating into their constituent ions.
Hydrogen Chloride (HCl)
Hydrogen chloride is a strong acid, widely used in industrial processes and laboratory settings. It is a crucial component in the production of various chemicals. Its aqueous solution is known as hydrochloric acid.
Hypochlorites
Hypochlorites, such as sodium hypochlorite (NaClO), are powerful oxidizing agents used as bleaches and disinfectants. Their effectiveness in killing microorganisms makes them invaluable in water treatment and sanitation.
Chlorates and Perchlorates
Chlorates and perchlorates are oxygen-containing chlorine compounds with high oxidation states. They find use as oxidizers in various applications, but their environmental impact requires careful management.
Conclusion: The Importance of Chlorine
Chlorine, despite its potential hazards, remains an incredibly important element with a wide range of applications that profoundly impact our daily lives. From the water we drink to the plastics we use, chlorine plays a significant role in modern society. Understanding its properties, uses, and potential environmental impacts is essential for responsible and sustainable use of this versatile element. Ongoing research continues to refine its applications and minimize its negative impacts, ensuring its continued importance in the future. The careful management and responsible use of chlorine are crucial for both human health and environmental protection. Further research into environmentally friendly alternatives and safer handling procedures will continue to shape the future of chlorine use.
Latest Posts
Latest Posts
-
How Far Is 1 4 Mile
Mar 18, 2025
-
How Many Phosphate Groups Does Atp Have
Mar 18, 2025
-
Is The Number 31 Prime Or Composite
Mar 18, 2025
-
Highest Common Factor Of 24 And 28
Mar 18, 2025
-
What Is 35 As A Fraction
Mar 18, 2025
Related Post
Thank you for visiting our website which covers about What Is Cl In The Periodic Table . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
