What Is The Mass Number Of An Isotope Equal To
listenit
Mar 22, 2025 · 6 min read
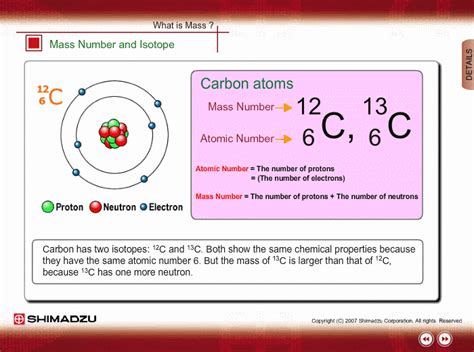
Table of Contents
What is the Mass Number of an Isotope Equal To? A Deep Dive into Isotopes and Atomic Mass
Understanding isotopes and their mass numbers is fundamental to grasping the intricacies of chemistry and nuclear physics. This comprehensive guide will delve deep into the concept of isotopes, explaining what their mass numbers represent and how they are determined. We will also explore the relationship between mass number, atomic number, and the number of neutrons, along with real-world applications and implications.
Understanding Isotopes: The Building Blocks of Matter
Before we delve into mass numbers, let's establish a clear understanding of isotopes themselves. Atoms, the fundamental units of matter, are composed of three subatomic particles: protons, neutrons, and electrons. Protons carry a positive charge and reside within the atom's nucleus. Neutrons, as their name suggests, are electrically neutral and also reside in the nucleus. Electrons, which carry a negative charge, orbit the nucleus.
The atomic number of an element is defined by the number of protons in its nucleus. This number is unique to each element and determines its chemical properties. For example, hydrogen (H) has an atomic number of 1, meaning it has one proton in its nucleus. Oxygen (O) has an atomic number of 8, indicating eight protons.
Now, here's where isotopes come into play. Isotopes are atoms of the same element that have the same atomic number (number of protons) but differ in their number of neutrons. This difference in neutron number affects the atom's mass but not its chemical behavior.
Examples of Isotopes:
- Carbon: Carbon-12 (¹²C) has 6 protons and 6 neutrons. Carbon-13 (¹³C) has 6 protons and 7 neutrons. Carbon-14 (¹⁴C) has 6 protons and 8 neutrons. All three are isotopes of carbon, sharing the same atomic number (6) but varying in their neutron count and, consequently, their mass.
- Hydrogen: Hydrogen has three isotopes: protium (¹H), deuterium (²H), and tritium (³H). Protium has one proton and zero neutrons, deuterium has one proton and one neutron, and tritium has one proton and two neutrons.
Defining Mass Number: The Sum of Protons and Neutrons
The mass number of an isotope is simply the total number of protons and neutrons in its nucleus. It's represented by a superscript to the left of the element's symbol. Therefore, the mass number directly reflects the isotope's atomic mass, expressed in atomic mass units (amu). One amu is approximately the mass of a single proton or neutron.
Mass Number = Number of Protons + Number of Neutrons
Let's revisit our carbon isotopes:
- ¹²C: Mass number = 6 protons + 6 neutrons = 12 amu
- ¹³C: Mass number = 6 protons + 7 neutrons = 13 amu
- ¹⁴C: Mass number = 6 protons + 8 neutrons = 14 amu
As you can see, the mass number accurately reflects the total number of nucleons (protons and neutrons) within the isotope's nucleus. It's a crucial identifier for distinguishing between different isotopes of the same element.
Atomic Mass vs. Mass Number: A Key Distinction
While closely related, atomic mass and mass number are not interchangeable terms. The mass number is a whole number representing the sum of protons and neutrons in a specific isotope. Atomic mass, on the other hand, is the weighted average of the masses of all naturally occurring isotopes of an element. This weighted average takes into account the abundance of each isotope in nature.
For instance, the atomic mass of carbon is approximately 12.01 amu. This is not a whole number because it represents the average mass considering the relative abundances of ¹²C, ¹³C, and ¹⁴C. The mass number, however, is a whole number specific to each isotope.
Calculating Atomic Mass: A Weighted Average Approach
To calculate the atomic mass of an element, you need to know the mass number and relative abundance of each of its isotopes. The calculation involves multiplying the mass number of each isotope by its relative abundance (expressed as a decimal fraction), summing these products, and then expressing the final result in atomic mass units (amu).
For example, let's consider an element with two isotopes:
- Isotope 1: Mass number = 60 amu, Relative abundance = 69% (0.69)
- Isotope 2: Mass number = 62 amu, Relative abundance = 31% (0.31)
Atomic mass = (60 amu × 0.69) + (62 amu × 0.31) = 61.0 amu (approximately)
This weighted average calculation reflects the contribution of each isotope to the overall atomic mass of the element.
The Significance of Mass Number: Applications and Implications
The mass number of an isotope holds significant importance across various scientific fields:
1. Nuclear Chemistry and Physics:
- Nuclear reactions: Mass numbers are crucial in balancing nuclear equations, ensuring the conservation of mass and charge during nuclear processes such as radioactive decay, fission, and fusion.
- Radioactive dating: Isotopes with specific mass numbers, like ¹⁴C, are used in radiocarbon dating to determine the age of organic materials. The decay rate of ¹⁴C, with its known mass number, allows scientists to estimate the time elapsed since the organism's death.
- Nuclear medicine: Radioisotopes with specific mass numbers are used in medical imaging techniques, such as PET (positron emission tomography) scans, enabling visualization of internal organs and processes for diagnostic purposes.
- Nuclear power: Understanding the mass numbers of isotopes involved in nuclear fission (e.g., ²³⁵U) is critical for controlling and managing the energy released in nuclear power plants.
2. Chemistry and Biochemistry:
- Mass spectrometry: Mass spectrometry techniques rely heavily on mass numbers to identify and quantify different isotopes and molecules within a sample. The mass-to-charge ratio of ions is measured, allowing researchers to deduce their mass numbers and subsequently the composition of the sample.
- Isotope labeling: Isotopes with specific mass numbers can be used as tracers to track the movement and metabolism of molecules within biological systems.
3. Geology and Geochemistry:
- Geological dating: Similar to radiocarbon dating, isotopic analysis involving mass numbers helps determine the age of rocks and geological formations.
Beyond the Basics: Nuclear Stability and Isotope Abundance
The stability of an isotope is closely related to its neutron-to-proton ratio. Isotopes with specific neutron-proton ratios tend to be more stable than others. Isotopes that are unstable undergo radioactive decay, transforming into more stable isotopes. This decay releases energy in the form of radiation.
The relative abundance of isotopes within a natural sample reflects their stability and the processes that have shaped the sample's formation and history. More stable isotopes generally have higher abundances than less stable ones.
Conclusion: A Cornerstone of Atomic Understanding
The mass number of an isotope, a seemingly simple concept, serves as a foundational element in understanding the structure of matter and its behavior. Its significance extends across diverse scientific disciplines, highlighting its critical role in unraveling the mysteries of the atomic world and its profound impact on our lives. From medical applications to geological dating, the mass number provides a crucial piece of information that enables researchers and scientists to make groundbreaking discoveries and innovations. A thorough grasp of this fundamental concept is essential for anyone seeking a deeper understanding of chemistry, physics, and the natural world.
Latest Posts
Latest Posts
-
What Is The Volume Of A Basketball
May 09, 2025
-
What Is The Name Of Feo
May 09, 2025
-
Determine The Oxidation State Of C In Co3 2
May 09, 2025
-
22 Is 25 Of What Number
May 09, 2025
-
Can You Be Accurate But Not Precise
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Mass Number Of An Isotope Equal To . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.