What Is The Gram Formula Mass Of Nh4 3po4
listenit
Mar 19, 2025 · 5 min read
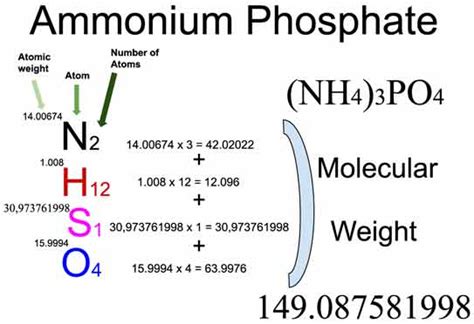
Table of Contents
What is the Gram Formula Mass of (NH₄)₃PO₄? A Deep Dive into Calculating and Understanding Molar Mass
Determining the gram formula mass (also known as molar mass) of a compound is a fundamental skill in chemistry. This article will delve into the calculation of the gram formula mass of ammonium phosphate, (NH₄)₃PO₄, explaining the process step-by-step and highlighting the importance of understanding molar mass in various chemical applications.
Understanding Gram Formula Mass (Molar Mass)
Before we tackle the specific calculation for (NH₄)₃PO₄, let's clarify the concept of gram formula mass. The gram formula mass is the mass of one mole of a substance. A mole is a unit in chemistry representing a specific number of particles (atoms, molecules, ions, etc.), defined as Avogadro's number, approximately 6.022 x 10²³. Therefore, the gram formula mass is essentially the mass in grams of 6.022 x 10²³ formula units of a compound.
The term "gram formula mass" is often used interchangeably with "molar mass," both referring to the same concept. We'll use both terms throughout this article to reinforce their equivalence.
Calculating the Molar Mass of (NH₄)₃PO₄
Ammonium phosphate, (NH₄)₃PO₄, is an ionic compound composed of ammonium ions (NH₄⁺) and phosphate ions (PO₄³⁻). To calculate its molar mass, we need to consider the molar mass of each element present in the compound and the number of atoms of each element.
Step 1: Identify the Elements and their Atomic Masses
(NH₄)₃PO₄ contains the elements nitrogen (N), hydrogen (H), phosphorus (P), and oxygen (O). We need to find the atomic mass of each element from the periodic table. The values may vary slightly depending on the source, but here are typical values:
- Nitrogen (N): 14.01 g/mol
- Hydrogen (H): 1.01 g/mol
- Phosphorus (P): 30.97 g/mol
- Oxygen (O): 16.00 g/mol
Step 2: Determine the Number of Atoms of Each Element
Let's break down the formula (NH₄)₃PO₄:
- Nitrogen (N): There are 3 ammonium ions (NH₄⁺), and each contains 1 nitrogen atom. Therefore, there are 3 × 1 = 3 nitrogen atoms.
- Hydrogen (H): Each ammonium ion (NH₄⁺) has 4 hydrogen atoms, and there are 3 ammonium ions. Therefore, there are 3 × 4 = 12 hydrogen atoms.
- Phosphorus (P): There is 1 phosphorus atom in the phosphate ion (PO₄³⁻).
- Oxygen (O): There are 4 oxygen atoms in the phosphate ion (PO₄³⁻).
Step 3: Calculate the Molar Mass
Now, we can calculate the molar mass by multiplying the atomic mass of each element by the number of atoms of that element and summing the results:
Molar Mass of (NH₄)₃PO₄ = (3 × Atomic Mass of N) + (12 × Atomic Mass of H) + (1 × Atomic Mass of P) + (4 × Atomic Mass of O)
Molar Mass of (NH₄)₃PO₄ = (3 × 14.01 g/mol) + (12 × 1.01 g/mol) + (1 × 30.97 g/mol) + (4 × 16.00 g/mol)
Molar Mass of (NH₄)₃PO₄ = 42.03 g/mol + 12.12 g/mol + 30.97 g/mol + 64.00 g/mol
Molar Mass of (NH₄)₃PO₄ = 149.12 g/mol
Therefore, the gram formula mass of ammonium phosphate, (NH₄)₃PO₄, is approximately 149.12 g/mol. This means that one mole of (NH₄)₃PO₄ weighs approximately 149.12 grams.
Importance of Molar Mass in Chemistry
The concept of molar mass is crucial in various chemical calculations and applications:
-
Stoichiometry: Molar mass is essential for converting between mass and moles in stoichiometric calculations. This allows chemists to determine the amounts of reactants and products involved in chemical reactions. For example, knowing the molar mass of (NH₄)₃PO₄ enables us to calculate how many grams are needed to obtain a specific number of moles for a reaction.
-
Solution Preparation: When preparing solutions of known concentration (e.g., molarity), the molar mass is used to calculate the mass of solute required to make a solution of a specific volume and concentration. For instance, if you need to prepare a 1M solution of (NH₄)₃PO₄, you'd use its molar mass to determine how many grams to dissolve in a liter of solvent.
-
Titrations: Molar mass plays a crucial role in titrations, where the concentration of an unknown solution is determined by reacting it with a solution of known concentration. The molar mass of the analyte and the titrant are used in calculations to determine the unknown concentration.
-
Gas Laws: The ideal gas law (PV = nRT) uses the number of moles (n) to relate pressure, volume, and temperature of a gas. Knowing the molar mass allows us to convert the mass of a gas to moles to utilize in the ideal gas law calculations.
-
Analytical Chemistry: Molar mass is often used in various analytical techniques, such as mass spectrometry and elemental analysis, to identify and quantify the components of a sample.
Applications of Ammonium Phosphate
Ammonium phosphate, (NH₄)₃PO₄, finds applications in various fields:
-
Fertilizers: It's a widely used fertilizer providing both nitrogen and phosphorus, essential nutrients for plant growth.
-
Food Industry: It can be used as a leavening agent in some food products.
-
Flame Retardants: Ammonium phosphate compounds are used in some fire-retardant materials.
-
Water Treatment: It can be used in water treatment to remove certain impurities.
Conclusion
Calculating the gram formula mass (molar mass) is a fundamental skill in chemistry, with broad applications across various fields. Understanding this concept is essential for performing accurate stoichiometric calculations, preparing solutions, conducting titrations, analyzing gases, and various other analytical procedures. The calculated molar mass of (NH₄)₃PO₄, approximately 149.12 g/mol, provides a crucial piece of information for anyone working with this important compound in chemistry, agriculture, or related disciplines. This value is used extensively in applications involving this fertilizer and in various laboratory settings requiring precise measurements and calculations. The detailed explanation provided here emphasizes the importance of careful calculation and an understanding of fundamental chemical principles.
Latest Posts
Latest Posts
-
What Is The Formula For Magnesium Sulfide
Mar 19, 2025
-
How Many Drops To A Ml
Mar 19, 2025
-
The Monomers Of Proteins Are Known As
Mar 19, 2025
-
What Percentage Is 2 Out Of 7
Mar 19, 2025
-
Is 87 A Composite Or Prime Number
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Gram Formula Mass Of Nh4 3po4 . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
