What Is The Electronic Configuration Of Chlorine
listenit
Mar 28, 2025 · 5 min read
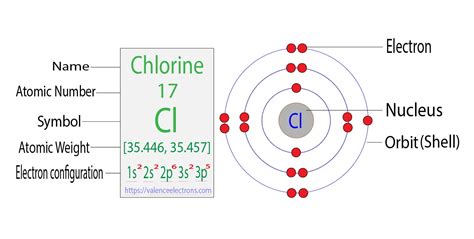
Table of Contents
What is the Electronic Configuration of Chlorine? A Deep Dive into Atomic Structure
Chlorine, a vital element crucial to life and various industrial processes, boasts a fascinating electronic configuration that dictates its chemical behavior and properties. Understanding this configuration is key to comprehending chlorine's reactivity, bonding patterns, and overall role in the natural world. This article will explore the electronic configuration of chlorine in detail, examining its underlying principles, implications, and exceptions.
Understanding Electronic Configuration
Before delving into chlorine's specific configuration, let's establish a foundational understanding of the concept. Electronic configuration describes the arrangement of electrons within the different energy levels and sublevels of an atom. This arrangement is governed by several fundamental principles:
- Aufbau Principle: Electrons fill orbitals in order of increasing energy levels. Lower energy levels are filled before higher ones.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, each with opposite spins.
- Hund's Rule: Electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion.
These principles guide us in predicting the electronic configuration of any element, including chlorine.
Determining Chlorine's Electronic Configuration
Chlorine (Cl) has an atomic number of 17, meaning it possesses 17 protons and, in its neutral state, 17 electrons. To determine its electronic configuration, we follow the Aufbau principle and fill the orbitals accordingly:
- 1s²: The first energy level (n=1) contains only the s subshell, which can hold up to two electrons.
- 2s²: The second energy level (n=2) also contains an s subshell, accommodating another two electrons.
- 2p⁶: The second energy level also includes the p subshell, which can hold up to six electrons (three orbitals, each with two electrons).
- 3s²: The third energy level (n=3) begins with the s subshell, holding two electrons.
- 3p⁵: Finally, the third energy level's p subshell receives the remaining five electrons.
Therefore, the complete electronic configuration of chlorine is 1s²2s²2p⁶3s²3p⁵. This configuration is often shorthand as [Ne]3s²3p⁵, where [Ne] represents the electronic configuration of neon (1s²2s²2p⁶), a noble gas with a stable octet.
Visualizing Chlorine's Electronic Structure
The electronic configuration can be visualized using various diagrams, including:
-
Orbital diagrams: These diagrams show each orbital as a box, with electrons represented by arrows. For chlorine, this would show fully filled 1s, 2s, 2p, and 3s orbitals, and a 3p subshell with one orbital fully filled and two orbitals each containing one electron.
-
Electron shell diagrams: These offer a simpler representation, showing electron shells as concentric circles. Chlorine would have two electrons in the inner shell, eight in the second shell, and seven in the outermost shell (valence shell).
These visual aids help in understanding the spatial distribution of electrons within the chlorine atom.
The Significance of Chlorine's Valence Electrons
The outermost shell, containing seven electrons (3s²3p⁵), is crucial in determining chlorine's chemical properties. These electrons are called valence electrons, and they participate directly in chemical bonding. Because chlorine has seven valence electrons, it is only one electron short of achieving a stable octet (eight electrons in its outermost shell), a configuration that resembles the noble gases and is associated with high stability. This drives chlorine's high reactivity.
Chlorine's Reactivity and Bonding
Chlorine's strong tendency to gain an electron to achieve a stable octet explains its high reactivity. It readily forms ionic bonds with metals, accepting an electron to form a chloride ion (Cl⁻), which has a stable octet. For example, the reaction between sodium (Na) and chlorine produces sodium chloride (NaCl), common table salt. Sodium donates an electron to chlorine, forming Na⁺ and Cl⁻ ions, which are held together by electrostatic attraction.
Chlorine can also form covalent bonds with other nonmetals, sharing electrons to achieve a stable octet. For example, in chlorine gas (Cl₂), two chlorine atoms share one pair of electrons, forming a single covalent bond. This completes the octet for both chlorine atoms.
Exceptions and Variations in Electronic Configuration
While the standard electronic configuration provides a good approximation, slight variations can occur in excited states or under specific conditions. For example, when chlorine absorbs energy, an electron might jump to a higher energy level, temporarily altering the configuration. However, these excited states are generally short-lived and return to the ground state configuration.
Chlorine's Role in the Natural World and Industry
Chlorine's unique electronic configuration translates to its diverse applications in various fields:
-
Water Purification: Chlorine's powerful oxidizing properties make it an effective disinfectant, widely used to purify drinking water and swimming pools. It kills harmful bacteria and viruses, ensuring safe water supplies.
-
Industrial Chemistry: Chlorine is a vital reagent in many industrial processes, including the production of plastics (PVC), solvents, and various other chemicals.
-
Medicine: Chlorine-containing compounds find applications in pharmaceuticals and medical treatments.
However, it's important to note that chlorine gas is highly toxic and corrosive, requiring careful handling and safety measures.
Applications and Further Research
The detailed understanding of chlorine's electronic configuration is fundamental to its many applications and its continued study. Ongoing research continues to explore chlorine's properties and potential uses. For example, exploring its role in different chemical environments could unveil new applications or lead to innovative technologies.
Conclusion: The Importance of Understanding Electronic Configuration
The electronic configuration of chlorine, 1s²2s²2p⁶3s²3p⁵, provides a blueprint for understanding its chemical behavior and reactivity. This configuration, along with the principles of atomic structure, explains chlorine's propensity for forming ionic and covalent bonds, its strong oxidizing power, and its diverse applications in various fields. This fundamental knowledge forms the foundation for deeper investigations into chlorine's properties and ongoing research to explore its potential applications. Its significance extends beyond simple chemical properties, playing a vital role in numerous natural processes and technological advances. From disinfecting drinking water to producing essential materials, understanding chlorine's electronic structure is crucial for harnessing its utility while acknowledging its inherent dangers. Therefore, comprehensive knowledge of the electronic configuration remains an essential pillar of chemistry and related scientific disciplines.
Latest Posts
Latest Posts
-
X 3 2x 2 X 4
Mar 31, 2025
-
How Is Magma Created In A Subduction Zone
Mar 31, 2025
-
What Is The Least Reactive Element
Mar 31, 2025
-
How To Subtract Negative And Positive Fractions
Mar 31, 2025
-
Find The Area Of A Triangle With Vertices
Mar 31, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electronic Configuration Of Chlorine . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
