What Is The Electronic Configuration Of Calcium Ca
listenit
Mar 20, 2025 · 5 min read
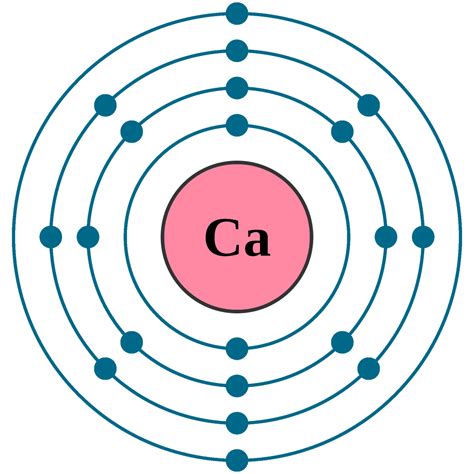
Table of Contents
What is the Electronic Configuration of Calcium (Ca)? A Deep Dive into Atomic Structure
Calcium, a vital element for life, plays a crucial role in various biological processes. Understanding its electronic configuration is key to grasping its chemical behavior and biological significance. This comprehensive guide delves into the electronic configuration of calcium (Ca), explaining the underlying principles and exploring its implications.
Understanding Electronic Configuration
The electronic configuration of an atom describes how electrons are arranged in its shells and subshells. This arrangement dictates the atom's chemical properties, its reactivity, and its ability to form bonds with other atoms. It's governed by the principles of quantum mechanics, specifically the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
The Aufbau Principle
The Aufbau principle, meaning "building-up" in German, states that electrons fill atomic orbitals in order of increasing energy levels. Lower energy levels are filled first before moving to higher energy levels. This sequential filling determines the electronic configuration.
Hund's Rule
Hund's rule dictates that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This maximizes the total spin of the electrons in the subshell, resulting in a more stable configuration.
The Pauli Exclusion Principle
The Pauli exclusion principle asserts that no two electrons in an atom can have the same set of four quantum numbers (principal, azimuthal, magnetic, and spin quantum numbers). This implies that each orbital can hold a maximum of two electrons, with opposite spins.
Determining the Electronic Configuration of Calcium (Ca)
Calcium (Ca) has an atomic number of 20, meaning it has 20 protons and 20 electrons in a neutral atom. To determine its electronic configuration, we follow the Aufbau principle, filling orbitals in increasing energy order.
The order of filling is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... However, some exceptions exist due to the subtle energy differences between orbitals.
For Calcium, the electronic configuration is straightforward:
1s² 2s² 2p⁶ 3s² 3p⁶ 4s²
Let's break this down:
- 1s²: The first energy level (n=1) contains the s subshell, which can hold a maximum of two electrons. Both electrons in Calcium occupy this orbital.
- 2s²: The second energy level (n=2) also has an s subshell, accommodating another two electrons.
- 2p⁶: The second energy level also contains a p subshell, which has three orbitals, each capable of holding two electrons. Therefore, the 2p subshell holds a total of six electrons.
- 3s²: The third energy level (n=3) begins with the s subshell, holding two more electrons.
- 3p⁶: Similar to 2p, the 3p subshell holds six electrons.
- 4s²: Finally, the fourth energy level (n=4) starts with the s subshell, accommodating the last two electrons of Calcium.
Valence Electrons and Chemical Reactivity
The valence electrons are the electrons in the outermost shell of an atom. These electrons are crucial in determining an atom's chemical behavior and its ability to form chemical bonds. In Calcium's case, the valence electrons are located in the 4s subshell.
Calcium has two valence electrons. This configuration explains why Calcium readily loses these two electrons to achieve a stable octet (a full outermost shell), similar to the noble gas Argon. This process forms a Ca²⁺ ion, a cation with a 2+ charge. This characteristic is what makes Calcium highly reactive, especially with electronegative elements like oxygen and chlorine.
Calcium's Role in Biological Systems
The chemical reactivity of Calcium, driven by its electronic configuration, is fundamental to its role in numerous biological processes:
- Bone and Teeth Formation: Calcium ions are essential components of the hydroxyapatite crystals that make up the mineral matrix of bones and teeth, providing structural strength and rigidity.
- Muscle Contraction: Calcium ions act as intracellular messengers, triggering muscle contraction by binding to proteins such as troponin, initiating a cascade of events that lead to muscle fiber shortening.
- Nerve Impulse Transmission: Calcium ions play a vital role in neurotransmission, facilitating the release of neurotransmitters at synapses, ensuring effective communication between nerve cells.
- Blood Clotting: Calcium ions are crucial cofactors in the blood clotting cascade, enabling the formation of blood clots to prevent excessive bleeding.
- Enzyme Activation: Many enzymes require Calcium ions as cofactors for their proper function, catalyzing biochemical reactions crucial for cellular processes.
- Cell Signaling: Calcium ions act as second messengers in numerous cellular signaling pathways, regulating gene expression, cell growth, and cell death.
Comparison with Other Elements
Understanding Calcium's electronic configuration allows for comparisons with other elements. For instance, Magnesium (Mg), with an atomic number of 12, has the electronic configuration 1s² 2s² 2p⁶ 3s². It also has two valence electrons, explaining its similar reactivity to Calcium, although generally less reactive.
On the other hand, Potassium (K), with an atomic number of 19, has the electronic configuration 1s² 2s² 2p⁶ 3s² 3p⁶ 4s¹. It possesses only one valence electron and therefore exhibits different chemical properties than Calcium, readily losing one electron to form a K⁺ ion.
Beyond the Basic Configuration: Excited States
While the ground state electronic configuration discussed above represents the most stable arrangement of electrons, Calcium, like other atoms, can exist in excited states. These states occur when an electron absorbs energy and jumps to a higher energy level. This results in a temporary, less stable electronic configuration. These excited states are involved in various processes such as atomic spectroscopy and light emission. For example, an electron from the 4s orbital might be promoted to a higher energy 3d or 4p orbital upon absorption of sufficient energy.
Conclusion: The Significance of Understanding Electronic Configuration
The electronic configuration of Calcium (Ca), 1s² 2s² 2p⁶ 3s² 3p⁶ 4s², is fundamental to understanding its chemical behavior, reactivity, and crucial biological roles. Its two valence electrons determine its propensity to form Ca²⁺ ions, enabling its involvement in vital processes like bone formation, muscle contraction, and nerve impulse transmission. This understanding extends beyond Calcium, providing a framework for comprehending the properties of other elements and their interactions. The principles of the Aufbau principle, Hund's rule, and the Pauli exclusion principle are fundamental to understanding atomic structure and chemical bonding. By understanding electronic configurations, we unlock a deeper understanding of the physical and biological world.
Latest Posts
Latest Posts
-
What Is The Gcf Of 39 And 26
Mar 20, 2025
-
What Is 2 1 3 As A Decimal
Mar 20, 2025
-
Integral Of 1 Y 2 1
Mar 20, 2025
-
What Percentage Of 80 Is 20
Mar 20, 2025
-
1 6 On A Number Line
Mar 20, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electronic Configuration Of Calcium Ca . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
