What Is The Electron Configuration Of Nickel
listenit
Mar 27, 2025 · 5 min read
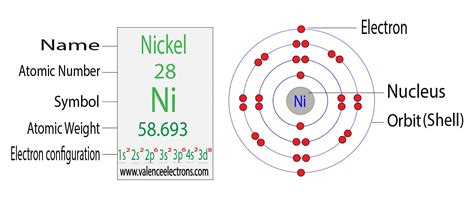
Table of Contents
What is the Electron Configuration of Nickel? A Deep Dive into Atomic Structure
Nickel, a silvery-white metal known for its strength and resistance to corrosion, holds a fascinating place in the periodic table. Understanding its atomic structure, particularly its electron configuration, is key to comprehending its unique properties and chemical behavior. This article delves deep into the electron configuration of nickel, exploring its implications and relating it to the broader context of atomic theory.
Understanding Electron Configuration
Before we dive into the specifics of nickel, let's establish a foundational understanding of electron configuration. An electron configuration describes the arrangement of electrons within the electron shells and subshells of an atom. This arrangement dictates how an atom will interact with other atoms, forming chemical bonds and determining its chemical and physical properties. It follows specific rules based on quantum mechanics, including the Aufbau principle, Hund's rule, and the Pauli exclusion principle.
The Aufbau Principle
The Aufbau principle, often described as the "building-up" principle, dictates that electrons fill atomic orbitals in order of increasing energy levels. Lower energy levels fill before higher ones. This principle guides us in predicting the electron configuration of an atom.
Hund's Rule
Hund's rule states that electrons will individually occupy each orbital within a subshell before doubling up in any one orbital. This minimizes electron-electron repulsion and leads to a more stable configuration. Each orbital within a subshell is first filled with a single electron before pairing begins.
The Pauli Exclusion Principle
The Pauli exclusion principle asserts that no two electrons in an atom can have the same set of four quantum numbers. This means that each orbital can hold a maximum of two electrons, and those two electrons must have opposite spins (represented as +1/2 and -1/2).
Determining the Electron Configuration of Nickel (Ni)
Nickel (Ni) has an atomic number of 28, meaning it possesses 28 protons and, in its neutral state, 28 electrons. To determine its electron configuration, we follow the Aufbau principle, filling orbitals in order of increasing energy.
The order of filling orbitals is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p... and so on.
Therefore, the full electron configuration of nickel is: 1s² 2s² 2p⁶ 3s² 3p⁶ 4s² 3d⁸.
Let's break this down:
- 1s²: Two electrons fill the 1s orbital.
- 2s²: Two electrons fill the 2s orbital.
- 2p⁶: Six electrons fill the three 2p orbitals (each holding two electrons).
- 3s²: Two electrons fill the 3s orbital.
- 3p⁶: Six electrons fill the three 3p orbitals.
- 4s²: Two electrons fill the 4s orbital. Note: Although the 3d orbitals are higher in energy than the 4s orbital in a filled state, the 4s orbital is filled before the 3d orbital.
- 3d⁸: Eight electrons partially fill the five 3d orbitals. According to Hund's rule, these eight electrons will occupy the five 3d orbitals individually before pairing up.
Noble Gas Configuration of Nickel
For simplicity, and to highlight the valence electrons, we can often express electron configurations using noble gas notation. Noble gases are group 18 elements with completely filled valence electron shells, resulting in exceptional stability. We use the noble gas preceding the element in the periodic table as a shorthand. For nickel, this is Argon (Ar), which has the electron configuration 1s² 2s² 2p⁶ 3s² 3p⁶.
Therefore, the noble gas configuration of nickel is: [Ar] 4s² 3d⁸. This notation clearly shows the electrons beyond the stable argon core.
Implications of Nickel's Electron Configuration
Nickel's electron configuration is directly responsible for its various chemical and physical properties:
Magnetic Properties:
The partially filled 3d orbitals are responsible for nickel's ferromagnetic properties. Ferromagnetism is a strong form of magnetism where the magnetic moments of unpaired electrons in neighboring atoms align, resulting in a strong overall magnetic field. The presence of unpaired electrons in the 3d orbitals is crucial to this. This property makes nickel valuable in various applications, from magnets and magnetic recording media to alloys with enhanced magnetic properties.
Catalytic Properties:
Nickel's electron configuration contributes to its catalytic activity. The partially filled d orbitals allow it to easily accept and donate electrons, facilitating chemical reactions. This is extensively utilized in industrial catalysis processes, including the hydrogenation of unsaturated fats and the production of ammonia (Haber-Bosch process).
Alloy Formation:
Nickel readily forms alloys with other metals due to its electronic configuration. The relatively accessible d-electrons facilitate strong metallic bonding with other metals, creating alloys with enhanced properties like strength, corrosion resistance, and specific magnetic characteristics. Examples include stainless steel and nickel-based superalloys used in high-temperature applications like jet engines.
Chemical Reactivity:
While not as reactive as alkali metals, nickel's partially filled d orbitals enable its participation in a variety of chemical reactions. It exhibits multiple oxidation states, meaning it can exist in various charged forms, each with differing chemical properties.
Variations and Excited States
While the ground state electron configuration ([Ar] 4s² 3d⁸) is the most stable and common, nickel can exist in excited states under specific conditions. These excited states involve the promotion of electrons to higher energy levels, typically from the 4s or 3d orbitals. These transitions are often observed in spectroscopic studies and are crucial for understanding nickel's interactions with light and its behavior in different chemical environments. The energy required to reach these excited states determines the wavelengths of light absorbed or emitted by the nickel atom, a phenomenon studied using techniques like atomic absorption spectroscopy.
Conclusion: Nickel's Electron Configuration in Context
The electron configuration of nickel, [Ar] 4s² 3d⁸, is not merely a set of numbers; it's a blueprint that dictates the atom's behavior and properties. This configuration explains its ferromagnetic properties, its catalytic capabilities, its alloy formation tendencies, and its diverse chemical reactivity. Understanding this configuration provides insight into nickel's wide range of applications, from everyday items to high-tech materials. The study of electron configuration is fundamental to understanding the periodic table's organization and the properties of all elements. Its study extends across various fields from material science and chemistry to physics and engineering. By mastering this concept, we gain a powerful tool for analyzing and predicting the behavior of matter at the atomic level.
Latest Posts
Latest Posts
-
Diameter Of Solar System In Light Years
Mar 30, 2025
-
Where Are The Nonmetals Located On The Periodic Table
Mar 30, 2025
-
How To Convert A Square Root To A Decimal
Mar 30, 2025
-
What Is The Percentage Of 12 Out Of 15
Mar 30, 2025
-
What Is The Gcf Of 25 And 15
Mar 30, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Nickel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
