What Is The Electron Configuration Of Li
listenit
Mar 23, 2025 · 6 min read
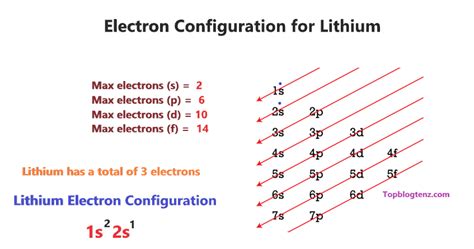
Table of Contents
What is the Electron Configuration of Li? A Deep Dive into Lithium's Atomic Structure
Lithium (Li), the lightest alkali metal, holds a significant place in both the periodic table and various applications, from batteries to medicine. Understanding its electronic structure, particularly its electron configuration, is key to grasping its unique chemical and physical properties. This comprehensive guide delves into the electron configuration of lithium, explaining its determination, significance, and implications.
Understanding Electron Configuration
Before diving into lithium's specifics, let's establish a foundational understanding of electron configuration. An electron configuration describes the arrangement of electrons in an atom's electron shells and subshells. This arrangement dictates how an atom will interact with other atoms, determining its chemical behavior and bonding characteristics. Electrons occupy specific energy levels, or shells, designated by principal quantum numbers (n = 1, 2, 3, etc.), where lower n values represent shells closer to the nucleus. Within each shell are subshells, denoted by s, p, d, and f, each capable of holding a specific number of electrons.
- The Aufbau Principle: Electrons fill orbitals starting from the lowest energy level and moving upward.
- Hund's Rule: Electrons individually occupy orbitals within a subshell before pairing up.
- Pauli Exclusion Principle: No two electrons in an atom can have the same four quantum numbers (n, l, ml, ms). This means each orbital can hold a maximum of two electrons with opposite spins.
These principles guide us in writing the electron configuration of any element.
Determining the Electron Configuration of Lithium (Li)
Lithium, with an atomic number of 3, possesses three protons and, in its neutral state, three electrons. Using the Aufbau principle, we systematically fill the electron shells and subshells:
-
The first shell (n=1): This shell contains only the s subshell, which can hold a maximum of two electrons. Therefore, the first two electrons of lithium occupy the 1s orbital.
-
The second shell (n=2): This shell contains both the s and p subshells. After filling the 1s orbital, the remaining electron occupies the 2s orbital.
Therefore, the complete electron configuration of lithium is 1s²2s¹. This concise notation tells us that the first shell (n=1) is completely filled with two electrons in the 1s orbital, while the second shell (n=2) contains one electron in the 2s orbital.
Visualizing Lithium's Electron Configuration
Imagine the nucleus of lithium at the center, surrounded by electron shells. The first shell, closest to the nucleus, holds two electrons tightly bound to the positive charge of the nucleus within the 1s orbital. The second shell, further away, houses the lone electron in the 2s orbital. This outer electron, being less tightly bound to the nucleus, is responsible for lithium's chemical reactivity.
Significance of Lithium's Electron Configuration
The 1s²2s¹ electron configuration is crucial in understanding lithium's properties:
-
Reactivity: The single electron in the 2s orbital is easily lost, making lithium highly reactive. It readily forms a +1 cation (Li⁺) by losing this electron to achieve a stable, filled electron shell configuration, mimicking the noble gas helium (He).
-
Alkaline Nature: Lithium's tendency to lose an electron and form a positive ion contributes to its alkaline nature. When reacted with water, it produces lithium hydroxide (LiOH), a strong base.
-
Bonding: Lithium's electron configuration dictates its bonding preferences. It primarily forms ionic bonds by transferring its valence electron to electronegative atoms like oxygen, chlorine, and fluorine.
-
Metallic Properties: While reactive, lithium also exhibits metallic properties due to the delocalized nature of its valence electron in the metallic lattice structure. This contributes to its good conductivity of heat and electricity.
-
Applications: Lithium's unique properties, rooted in its electron configuration, lead to its diverse applications:
- Lithium-ion batteries: The ease of lithium's electron transfer makes it ideal for rechargeable batteries, powering many electronic devices and electric vehicles.
- Medical applications: Lithium salts are used to treat certain mental health disorders, although the exact mechanisms are still under investigation.
- Lubricants: Lithium-based greases are used in high-temperature applications due to their thermal stability.
- Ceramics and glass: Lithium compounds are added to ceramics and glass to improve their properties.
Comparing Lithium's Electron Configuration to Other Elements
By comparing lithium's electron configuration to those of other elements, we can further understand periodic trends and relationships:
-
Helium (He): 1s²: Helium, a noble gas, has a completely filled first shell. This completely filled shell represents exceptional stability, explaining helium's inertness.
-
Beryllium (Be): 1s²2s²: Beryllium has two electrons in its outermost shell. It is less reactive than lithium because losing two electrons requires more energy.
-
Sodium (Na): 1s²2s²2p⁶3s¹: Sodium, like lithium, has one electron in its outermost shell. They both belong to Group 1 (alkali metals) and exhibit similar chemical properties, although sodium is more reactive than lithium due to the larger atomic size and weaker hold on its valence electron.
Advanced Concepts and Considerations
While the basic electron configuration provides a good understanding of lithium's behavior, more advanced concepts provide a more nuanced perspective:
-
Orbital Diagrams: These visual representations show the arrangement of electrons within individual orbitals, explicitly illustrating electron pairing and spin. For lithium, the orbital diagram would show two electrons paired in the 1s orbital and one unpaired electron in the 2s orbital.
-
Quantum Numbers: A complete description of an electron's state requires four quantum numbers: the principal quantum number (n), the azimuthal quantum number (l), the magnetic quantum number (ml), and the spin quantum number (ms). These numbers specify the energy level, subshell, orbital, and spin of each electron.
-
Effective Nuclear Charge: The effective nuclear charge experienced by an electron is the net positive charge after considering the shielding effect of inner electrons. In lithium, the 2s electron experiences a lower effective nuclear charge than the 1s electrons due to the shielding effect of the 1s electrons.
-
Ionization Energy: The energy required to remove an electron from an atom is known as ionization energy. Lithium has a relatively low first ionization energy due to its single valence electron being loosely held. However, the second ionization energy is significantly higher because it requires removing an electron from a stable, filled shell.
Conclusion
The seemingly simple electron configuration of lithium, 1s²2s¹, holds the key to understanding its diverse and fascinating properties. Its single valence electron dictates its reactivity, bonding behavior, and ultimate applications. By understanding the fundamental principles of electron configuration and applying them to lithium, we can appreciate the intricate connection between an atom's structure and its macroscopic properties. This knowledge is fundamental not only to chemistry but also to materials science, engineering, and various other fields reliant on the unique characteristics of this lightweight alkali metal. Further exploration into the nuances of quantum mechanics and atomic structure deepens our understanding even further, revealing the beauty and complexity hidden within the seemingly simple arrangement of electrons within the lithium atom.
Latest Posts
Latest Posts
-
What Do Letters Dna Stand For
Mar 25, 2025
-
What Is The Gcf Of 48 And 24
Mar 25, 2025
-
Molarity Of Acetic Acid In Vinegar
Mar 25, 2025
-
How Many Minutes Are In 1 Week
Mar 25, 2025
-
How Many 3rds In A Cup
Mar 25, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Li . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
