What Is The Electron Configuration Of Aluminum
listenit
Mar 18, 2025 · 5 min read
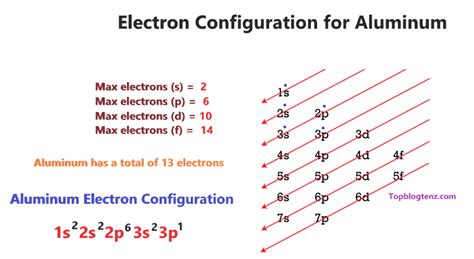
Table of Contents
What is the Electron Configuration of Aluminum? A Deep Dive
Aluminum, a lightweight yet incredibly strong metal, plays a crucial role in countless applications, from beverage cans to aerospace engineering. Understanding its properties begins with grasping its atomic structure, specifically its electron configuration. This article delves deep into the electron configuration of aluminum, exploring its implications for the element's chemical behavior and physical properties.
Understanding Electron Configuration
Before we dive into aluminum's specifics, let's establish a foundational understanding of electron configuration. An electron configuration describes how electrons are arranged in the various energy levels and sublevels within an atom. This arrangement dictates an atom's chemical reactivity and its position within the periodic table.
The arrangement follows specific rules:
- Aufbau Principle: Electrons fill the lowest energy levels first.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons with opposite spins.
- Hund's Rule: Electrons will individually occupy each orbital within a subshell before doubling up in any one orbital.
These principles guide us in predicting the electron configuration of any element, including aluminum.
Energy Levels and Sublevels
Electrons reside in energy levels (also called shells), denoted by the principal quantum number (n), where n = 1, 2, 3, and so on. Each energy level contains sublevels (also called subshells), designated as s, p, d, and f. These sublevels have different shapes and can hold a specific number of electrons:
- s sublevel: Holds a maximum of 2 electrons.
- p sublevel: Holds a maximum of 6 electrons.
- d sublevel: Holds a maximum of 10 electrons.
- f sublevel: Holds a maximum of 14 electrons.
The order of filling these sublevels is crucial and isn't always strictly sequential due to variations in energy levels. The general order is: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, 5s, 4d, 5p, 6s, 4f, 5d, 6p, 7s, 5f, 6d, 7p...
Determining the Electron Configuration of Aluminum (Al)
Aluminum has an atomic number of 13, meaning it has 13 protons and 13 electrons in a neutral atom. To determine its electron configuration, we follow the Aufbau principle and fill the sublevels according to the rules mentioned above:
- 1s²: The first energy level (n=1) has one s sublevel, which can hold two electrons. These two electrons fill the 1s orbital.
- 2s²: The second energy level (n=2) starts with the 2s sublevel, also holding two electrons.
- 2p⁶: The second energy level also contains a 2p sublevel, capable of holding six electrons. These six electrons fill the three 2p orbitals.
- 3s¹: After filling the 2p sublevel, we move to the third energy level (n=3), starting with the 3s sublevel. Aluminum only has one more electron remaining, which occupies the 3s orbital.
Therefore, the complete electron configuration of aluminum is 1s²2s²2p⁶3s¹.
Shorthand Notation
While the full electron configuration is accurate and informative, a shorthand notation using the noble gas configuration can simplify it. The noble gas that precedes aluminum in the periodic table is neon (Ne), which has the electron configuration 1s²2s²2p⁶. We can use this as a shorthand:
[Ne]3s¹
This notation effectively communicates the electron configuration by indicating that aluminum's electrons are arranged similarly to neon's, with one additional electron in the 3s orbital of the third energy level.
Implications of Aluminum's Electron Configuration
Aluminum's electron configuration has profound implications for its chemical and physical properties:
Chemical Reactivity:
The single electron in the 3s orbital is relatively loosely bound to the aluminum atom. This makes aluminum readily willing to lose this electron to achieve a stable octet (eight electrons) in its outermost shell, similar to the noble gas neon. This tendency to lose an electron makes aluminum highly reactive, particularly with oxidizing agents like oxygen. This explains why aluminum readily forms compounds like aluminum oxide (Al₂O₃), a protective layer that prevents further oxidation.
Oxidation State:
Because of its willingness to lose one electron, aluminum typically exhibits a +3 oxidation state in its compounds. This means that in chemical reactions, aluminum atoms lose three electrons, resulting in a +3 charge.
Physical Properties:
The electron configuration also influences aluminum's physical properties. The relatively weak attraction between the valence electron and the nucleus contributes to aluminum's low density and excellent electrical conductivity. The delocalized valence electrons are easily mobilized when an electric field is applied, allowing for efficient charge transport.
Aluminum's Role in Different Fields
Understanding aluminum's electron configuration allows us to appreciate its diverse applications:
Packaging:
Aluminum's resistance to corrosion and its malleability make it ideal for packaging materials, such as beverage cans and food containers. The protective oxide layer prevents food spoilage and keeps the contents fresh.
Transportation:
Aluminum's lightweight nature yet high strength-to-weight ratio makes it a favored material in the automotive and aerospace industries. It contributes to fuel efficiency in vehicles and reduces the weight of aircraft, improving performance and reducing fuel consumption.
Construction:
Aluminum alloys are used extensively in building construction, offering durability, lightweight characteristics, and corrosion resistance. They are used in windows, doors, roofing, and structural components.
Electrical Applications:
Aluminum's excellent electrical conductivity makes it essential in electrical wiring, transmission lines, and other electrical components. It's a cost-effective alternative to copper in many applications.
Conclusion:
The seemingly simple electron configuration of aluminum – 1s²2s²2p⁶3s¹ or [Ne]3s¹ – holds the key to understanding its remarkable properties and its widespread use in various industries. Its tendency to lose a single electron, resulting in a +3 oxidation state, dictates its chemical reactivity and its formation of stable compounds. The relatively weak binding of the valence electrons also contributes significantly to its physical properties, such as low density and high electrical conductivity. Understanding the electron configuration provides a fundamental basis for appreciating aluminum's vital role in modern technology and everyday life. The interplay between electronic structure and macroscopic properties highlights the power of understanding fundamental chemistry in predicting and manipulating material behavior.
Latest Posts
Latest Posts
-
How Many Atoms Are In A Door Per Cubic Meter
Mar 19, 2025
-
1 In 7 As A Percentage
Mar 19, 2025
-
Practice Problems For Completing The Square
Mar 19, 2025
-
Is Ice Melts A Chemical Change
Mar 19, 2025
-
How Many Pounds Is A Quarter Ton
Mar 19, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration Of Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
