Is Ice Melts A Chemical Change
listenit
Mar 19, 2025 · 5 min read
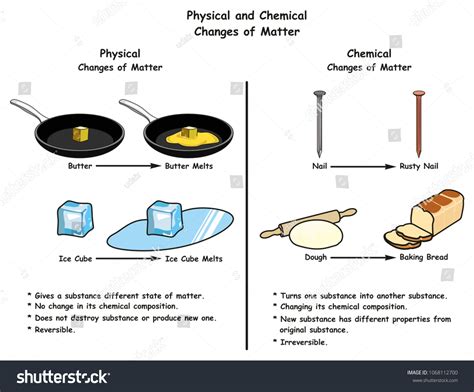
Table of Contents
Is Melting Ice a Chemical Change? A Deep Dive into Physical vs. Chemical Transformations
The question of whether melting ice represents a chemical change or a physical change is a fundamental one in understanding the nature of matter and its transformations. While seemingly simple, the answer requires a thorough understanding of the differences between chemical and physical changes and a closer look at the molecular structure of water. This article will delve into this topic, exploring the intricacies of phase transitions and debunking common misconceptions.
Understanding Chemical and Physical Changes
Before addressing the ice melting conundrum, let's establish a clear definition of chemical and physical changes.
Physical changes alter the form or appearance of a substance but do not change its chemical composition. No new substance is formed. Examples include changes in state (melting, freezing, boiling, condensation, sublimation), dissolving, and changes in shape or size. The original substance retains its fundamental chemical identity.
Chemical changes, also known as chemical reactions, involve the rearrangement of atoms to form new substances with different properties. These changes are often irreversible and involve the breaking and forming of chemical bonds. Examples include burning, rusting, cooking, and digestion. The products of a chemical reaction are fundamentally different from the reactants.
The Molecular Dance: Water's Phase Transitions
Water, in its various states – solid (ice), liquid (water), and gas (water vapor) – is composed of the same molecules: H₂O. Each molecule consists of two hydrogen atoms covalently bonded to a single oxygen atom. The key difference lies in the arrangement and interactions of these molecules.
-
Ice (Solid): In ice, water molecules are arranged in a highly ordered crystalline structure, held together by hydrogen bonds – relatively weak intermolecular forces. This structure results in the characteristic rigidity and low density of ice.
-
Liquid Water: Upon melting, the hydrogen bonds weaken, allowing the water molecules to move more freely. The crystalline structure breaks down, resulting in a less ordered arrangement. The molecules are still close together, but they can slide past each other, leading to the fluidity of liquid water.
-
Water Vapor (Gas): When water boils, the molecules gain enough kinetic energy to overcome the intermolecular forces entirely, becoming widely dispersed and independent in the gaseous phase.
Why Melting Ice is a Physical Change
The key to understanding why melting ice is a physical change lies in the fact that the chemical composition of the water molecules remains unchanged throughout the process. The chemical bonds within the H₂O molecules themselves are not broken or formed. Only the intermolecular forces (hydrogen bonds) between the molecules are affected.
No new substance is created. The ice melts into liquid water, which is still composed of H₂O molecules. You can freeze the liquid water back into ice, demonstrating the reversibility of the process, a hallmark of physical changes.
The chemical properties of water remain the same. The melting point, boiling point, and other chemical properties of the water remain unchanged. This further confirms that no new substance has been formed.
Addressing Common Misconceptions
Several misunderstandings often arise regarding the nature of melting ice:
Misconception 1: A change in state always implies a chemical change. This is incorrect. Phase transitions, such as melting, freezing, boiling, and condensation, are classic examples of physical changes.
Misconception 2: Energy absorption implies a chemical change. While chemical reactions often involve energy changes (exothermic or endothermic), energy absorption or release alone does not define a chemical change. Melting ice is an endothermic process (it absorbs heat), but this heat is used to overcome the intermolecular forces, not to break chemical bonds.
Misconception 3: The change in appearance signifies a chemical transformation. The visible transformation from solid ice to liquid water is a result of the rearrangement of molecules, not the creation of new ones. The change in appearance is a consequence of the physical change, not the defining characteristic.
Beyond the Basics: Exploring Deeper Implications
The seemingly straightforward process of melting ice provides a valuable entry point into more complex concepts in chemistry and physics. Understanding phase transitions allows us to grasp:
-
The role of intermolecular forces: Hydrogen bonding is crucial in determining the properties of water and many other substances. Understanding its influence is fundamental to comprehending the behavior of matter.
-
The concept of enthalpy: The heat absorbed during melting (enthalpy of fusion) is a critical thermodynamic property that governs phase transitions.
-
The importance of temperature and pressure: Changes in temperature and pressure can affect the melting point of ice, highlighting the interplay of different physical parameters.
-
Applications in various fields: Understanding phase transitions is crucial in diverse fields, including meteorology (weather patterns), material science (designing materials with specific properties), and even biology (cellular processes).
Conclusion: Melting Ice - A Quintessential Physical Change
In conclusion, melting ice is unequivocally a physical change. The process involves only a change in the physical state of water, driven by the weakening and breaking of intermolecular hydrogen bonds. The chemical composition of the water molecules remains unaltered throughout the transition. While seemingly simple, understanding this fundamental concept provides a solid foundation for exploring more complex chemical and physical phenomena. The seemingly simple act of ice melting provides a perfect illustration of the beautiful and fundamental principles governing the physical world. This deep dive into the process allows us to appreciate the intricacies of matter and its transformations, solidifying our understanding of the distinctions between physical and chemical changes. The seemingly simple process reveals the profound interconnectedness of chemistry, physics, and the world around us.
Latest Posts
Latest Posts
-
What Is The Frequency Of The Wave Shown Below
May 09, 2025
-
How To Simplify Square Root Of 80
May 09, 2025
-
How Many Orbitals Are There In The Third Shell
May 09, 2025
-
How Are Pressure And Volume Of A Gas Related
May 09, 2025
-
A Compound Held Together By Ionic Bonds Is Called
May 09, 2025
Related Post
Thank you for visiting our website which covers about Is Ice Melts A Chemical Change . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.