What Is The Electron Configuration For Neon
listenit
Mar 21, 2025 · 6 min read
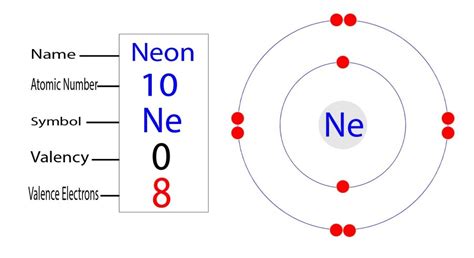
Table of Contents
What is the Electron Configuration for Neon? A Deep Dive into Atomic Structure
Neon, the vibrant gas that illuminates our signs and plays a crucial role in various technologies, holds a fascinating place in the periodic table. Understanding its electron configuration is key to unlocking its unique properties and behavior. This article delves deep into the electron configuration of neon, explaining the underlying principles, its significance, and how it relates to the element's overall characteristics.
Understanding Electron Configuration
Before we dive into neon's specific configuration, let's establish a foundation. Electron configuration describes the arrangement of electrons in the different energy levels (shells) and sublevels (subshells) within an atom. This arrangement dictates an atom's chemical properties, reactivity, and how it interacts with other atoms. The configuration is written using a shorthand notation, indicating the principal quantum number (n), the subshell (s, p, d, f), and the number of electrons in each subshell.
Principal Quantum Number (n)
The principal quantum number represents the energy level or shell of an electron. The lower the value of 'n', the closer the electron is to the nucleus and the lower its energy. The possible values for 'n' are positive integers (1, 2, 3, and so on).
Subshells (s, p, d, f)
Within each principal energy level (except for n=1), there are subshells. These subshells represent different regions of space where electrons are likely to be found. They are identified by the letters s, p, d, and f, each with a specific capacity for electrons:
- s subshell: Holds a maximum of 2 electrons.
- p subshell: Holds a maximum of 6 electrons.
- d subshell: Holds a maximum of 10 electrons.
- f subshell: Holds a maximum of 14 electrons.
The Aufbau Principle and Hund's Rule
Two fundamental principles guide the filling of electrons into subshells:
-
Aufbau Principle: Electrons fill the lowest energy levels first. This means that the 1s subshell is filled before the 2s, the 2s before the 2p, and so on.
-
Hund's Rule: Within a subshell, electrons will individually occupy each orbital before doubling up in any one orbital. This minimizes electron-electron repulsion. Each orbital within a subshell can hold a maximum of two electrons with opposite spins.
Neon's Electron Configuration: A Step-by-Step Explanation
Neon (Ne) has an atomic number of 10, meaning it has 10 protons in its nucleus and, in a neutral atom, 10 electrons orbiting it. Let's build up its electron configuration step-by-step, following the Aufbau principle and Hund's Rule:
-
1s²: The first energy level (n=1) has only an s subshell, which can hold a maximum of 2 electrons. These two electrons fill the 1s subshell completely.
-
2s²: The second energy level (n=2) starts with the 2s subshell, which also holds a maximum of 2 electrons. These fill next.
-
2p⁶: Finally, we reach the 2p subshell, which can hold up to 6 electrons. All six electrons fill this subshell.
Therefore, the complete electron configuration for neon is 1s²2s²2p⁶.
Significance of Neon's Electron Configuration
Neon's full outermost electron shell (2s²2p⁶) is the key to understanding its properties. A completely filled outermost shell, also known as a valence shell, makes neon exceptionally stable and unreactive. This is because a full valence shell represents a state of minimal energy, and atoms tend towards the lowest energy state possible. This stability is why neon is a noble gas – it rarely forms chemical bonds with other elements.
Noble Gases and Chemical Inertness
The noble gases (Helium, Neon, Argon, Krypton, Xenon, Radon) all share a common characteristic: they have completely filled valence shells. This results in their extreme chemical inertness. They rarely participate in chemical reactions, making them invaluable in applications where non-reactivity is crucial.
Applications of Neon and its Inertness
Neon's inertness and unique properties contribute to a wide range of applications:
-
Neon lighting: Neon gas, when energized electrically, emits a characteristic bright orange-red glow. This forms the basis of classic neon signs, still widely used for advertising and decorative purposes. While many "neon" signs use other gases to produce different colors, the name persists.
-
Laser technology: Neon is utilized in certain types of lasers, specifically He-Ne lasers (Helium-Neon lasers), which produce a coherent and monochromatic red light beam. These lasers are used in various applications including barcode scanners, laser pointers, and scientific research.
-
Cryogenics: Liquid neon, a very cold substance, is employed as a cryogenic refrigerant in scientific and industrial applications where extremely low temperatures are required.
-
Diving gas mixtures: In specialized diving applications, neon is sometimes used as a component in breathing gas mixtures to reduce nitrogen narcosis at significant depths. Its inertness makes it a safe choice for this purpose.
Beyond the Basic Configuration: Orbital Diagrams and Electron Spin
While the electron configuration provides a concise overview of electron arrangement, a more detailed picture involves orbital diagrams and considerations of electron spin.
Orbital Diagrams
Orbital diagrams visually represent the arrangement of electrons within individual orbitals. Each orbital can hold a maximum of two electrons with opposite spins (represented by arrows pointing up and down). For neon, the orbital diagram would show:
- 1s orbital: ↑↓
- 2s orbital: ↑↓
- 2p orbitals: ↑↓ ↑↓ ↑↓
Electron Spin
Electrons possess an intrinsic property called spin, which can be either "spin up" (+1/2) or "spin down" (-1/2). Hund's Rule dictates that electrons will first fill orbitals singly with parallel spins before pairing up with opposite spins. This minimizes electron-electron repulsion.
Neon and the Periodic Table
Neon's position in the periodic table (Group 18, Period 2) directly reflects its electron configuration. Its placement in Group 18, the noble gases, highlights its full valence shell and inherent inertness. Its position in Period 2 indicates that its outermost electrons are in the second principal energy level (n=2).
Comparison with Other Elements
Comparing neon's electron configuration with other elements reveals the periodic trends in chemical behavior. Elements with similar valence electron configurations often exhibit similar chemical properties. For instance, other noble gases like Argon (1s²2s²2p⁶3s²3p⁶) also possess a full valence shell and are chemically inert.
Conclusion: Neon's Configuration and its Significance
The electron configuration of neon, 1s²2s²2p⁶, is not just a symbolic representation; it's a fundamental description of the atom's structure and behavior. This configuration, with its completely filled valence shell, dictates neon's exceptional stability and inertness, qualities that are exploited in various technological applications, from bright neon signs to sophisticated laser technology. Understanding neon's electron configuration provides a crucial foundation for comprehending the properties and behavior of this fascinating element and its role in the world around us. The principles of electron configuration apply to all elements, allowing us to predict and explain their chemical reactivity and the properties that make them unique.
Latest Posts
Latest Posts
-
What Is Another Name For Newtons First Law
Mar 28, 2025
-
Earth Would Not Have Seasons If It
Mar 28, 2025
-
What Is The Least Common Multiple Of 3 9 15
Mar 28, 2025
-
How Do Stars Burn Without Oxygen
Mar 28, 2025
-
What Is 11 25 As A Percent
Mar 28, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Neon . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
