What Is The Electron Configuration For Aluminum
listenit
Mar 16, 2025 · 5 min read
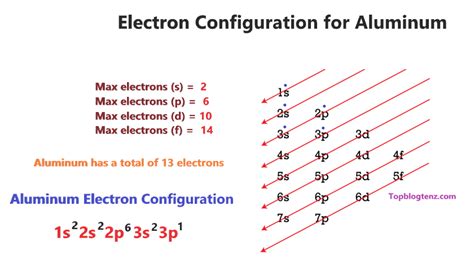
Table of Contents
What is the Electron Configuration for Aluminum? A Deep Dive into Atomic Structure
Aluminum, a lightweight yet strong metal ubiquitous in everyday life, holds a fascinating place in the periodic table. Understanding its properties begins with understanding its atomic structure, particularly its electron configuration. This article will delve deep into the electron configuration of aluminum, exploring the underlying principles of electron arrangement and how this configuration dictates aluminum's chemical behavior. We'll also explore related concepts like orbitals, quantum numbers, and the periodic trends that influence electron placement.
Understanding Electron Configuration
Electron configuration describes the arrangement of electrons in the different energy levels and sublevels within an atom. It's a fundamental concept in chemistry, crucial for understanding an element's reactivity, bonding capabilities, and physical properties. Electrons occupy specific energy levels, often depicted as shells surrounding the nucleus. Each shell comprises subshells, designated as s, p, d, and f, which themselves contain atomic orbitals. These orbitals are regions of space where there's a high probability of finding an electron.
The filling of these orbitals follows specific rules:
- Aufbau Principle: Electrons fill orbitals starting from the lowest energy level to the highest. This means filling 1s before 2s, 2s before 2p, and so on.
- Pauli Exclusion Principle: Each orbital can hold a maximum of two electrons, and these electrons must have opposite spins (represented as +1/2 and -1/2).
- Hund's Rule: Within a subshell (like 2p), electrons will individually occupy each orbital before doubling up in any one orbital. This minimizes electron-electron repulsion.
Determining Aluminum's Electron Configuration
Aluminum (Al) has an atomic number of 13, meaning it has 13 protons and, in a neutral atom, 13 electrons. To determine its electron configuration, we follow the Aufbau principle and the rules mentioned above.
The order of filling orbitals is generally: 1s, 2s, 2p, 3s, 3p, 4s, 3d, 4p, and so on. Let's fill the orbitals with Aluminum's 13 electrons:
- 1s²: The first shell (n=1) contains only the 's' subshell, which can hold up to two electrons. We fill it completely.
- 2s²: The second shell (n=2) also has an 's' subshell, holding another two electrons.
- 2p⁶: The second shell also includes a 'p' subshell, which can hold up to six electrons (three orbitals, each holding two electrons). We fill this completely.
- 3s²: Moving to the third shell (n=3), we fill the 's' subshell with two electrons.
- 3p¹: Finally, we place the remaining electron in the 'p' subshell of the third shell.
Therefore, the complete electron configuration for aluminum is 1s²2s²2p⁶3s²3p¹.
Orbital Diagrams and Quantum Numbers
Representing the electron configuration using an orbital diagram provides a more visual understanding. Each orbital is represented by a box, and electrons are shown as arrows within the boxes. For example:
1s: ↑↓ 2s: ↑↓ 2px: ↑↓ 2py: ↑↓ 2pz: ↑↓ 3s: ↑↓ 3px: ↑ 3py: 3pz:
This diagram visually reinforces the Pauli Exclusion Principle and Hund's Rule.
Quantum numbers provide a more detailed description of each electron's state within an atom. Four quantum numbers describe each electron:
- Principal Quantum Number (n): This describes the energy level or shell (n = 1, 2, 3...). For Aluminum's outermost electron, n = 3.
- Azimuthal Quantum Number (l): This describes the subshell (l = 0 for s, 1 for p, 2 for d, 3 for f). For Aluminum's outermost electron, l = 1 (p subshell).
- Magnetic Quantum Number (ml): This describes the specific orbital within the subshell (ml = -l, -l+1...0...l-1, l). For the outermost electron, ml could be -1, 0, or +1, depending on which 3p orbital it occupies.
- Spin Quantum Number (ms): This describes the electron's spin (+1/2 or -1/2).
Aluminum's Chemical Behavior and Electron Configuration
Aluminum's electron configuration explains its chemical behavior. The outermost electrons, located in the 3s and 3p orbitals, are its valence electrons. These electrons are the ones most involved in chemical bonding. Aluminum has three valence electrons, making it highly reactive. It readily loses these three electrons to achieve a stable octet (eight electrons in its outermost shell), forming a 3+ ion (Al³⁺). This explains why aluminum readily forms ionic compounds with elements like chlorine (AlCl₃) and oxygen (Al₂O₃). It also participates in metallic bonding, contributing to its characteristic malleability and ductility.
Comparing Aluminum's Configuration to Other Elements
Comparing aluminum's electron configuration to other elements in the same period and group provides valuable insights into periodic trends. For example:
- Across the period: As we move across period 3 from sodium (Na) to argon (Ar), the number of electrons and the number of protons increases, resulting in a progressively greater nuclear charge and a decrease in atomic radius. The addition of electrons to the same shell also leads to increased shielding, slightly offsetting the increased nuclear charge.
- Down the group: Comparing aluminum to other elements in Group 13 (boron, gallium, indium, thallium), we observe an increase in atomic radius and a decrease in ionization energy as we move down the group. The increased number of electron shells leads to greater shielding of the outer electrons from the nucleus.
Advanced Concepts: Electron Configuration Exceptions
While the Aufbau principle generally predicts electron configurations accurately, there are exceptions. These exceptions often occur in transition metals and inner transition metals due to the relatively small energy differences between the (n-1)d and ns orbitals. The electrons sometimes prefer to occupy the (n-1)d orbital to achieve a more stable half-filled or fully filled d subshell. Aluminum, however, doesn't exhibit such exceptions. Its electron configuration adheres strictly to the Aufbau principle.
Conclusion: The Significance of Aluminum's Electron Configuration
Understanding the electron configuration of aluminum is fundamental to grasping its chemical and physical properties. The arrangement of its 13 electrons, specifically its three valence electrons, directly determines its reactivity, bonding preferences, and the formation of its characteristic compounds. This knowledge is crucial in various fields, including materials science, chemistry, and engineering, where aluminum's unique properties are exploited in countless applications. The principles governing electron configuration extend beyond aluminum to all elements, providing a powerful framework for understanding the periodic table and the behavior of matter at the atomic level. By mastering these concepts, one gains a deeper appreciation for the intricate world of chemistry and the underlying structure that governs the properties of all elements.
Latest Posts
Latest Posts
-
How To Make Casio Calculator Show Scientific Notation
May 09, 2025
-
How Does An Air Regulator Work
May 09, 2025
-
Ground State Electron Configuration Of Al
May 09, 2025
-
Click On Each Chiral Center In The Cholesterol Derivative Below
May 09, 2025
-
Which Polar Is When They Are Attracted To Each Other
May 09, 2025
Related Post
Thank you for visiting our website which covers about What Is The Electron Configuration For Aluminum . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.