What Is The Difference Between Osmosis And Dialysis
listenit
Mar 17, 2025 · 6 min read
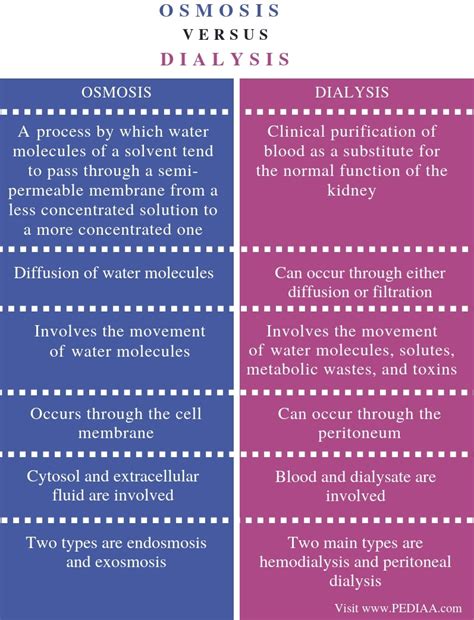
Table of Contents
What's the Difference Between Osmosis and Dialysis? A Deep Dive into Membrane Transport
Understanding the subtle yet crucial differences between osmosis and dialysis is fundamental to grasping various biological and chemical processes. While both involve the movement of substances across semi-permeable membranes, their mechanisms and driving forces differ significantly. This comprehensive guide delves into the intricacies of each process, highlighting their similarities and key distinctions.
Osmosis: The Movement of Water Across a Semi-Permeable Membrane
Osmosis is a specialized type of passive transport, driven by the difference in water potential across a selectively permeable membrane. In simpler terms, it's the movement of water molecules from a region of high water concentration (low solute concentration) to a region of low water concentration (high solute concentration) across a membrane that allows water to pass but restricts the movement of solutes.
Understanding Water Potential
Water potential is a measure of the tendency of water to move from one area to another. It's influenced by two main factors:
- Solute potential: This represents the effect of dissolved solutes on the water potential. The more solutes present, the lower the water potential. Think of it as the "pull" exerted by solutes on water molecules.
- Pressure potential: This refers to the physical pressure exerted on the water. Positive pressure (like turgor pressure in plant cells) increases water potential, while negative pressure (like tension in xylem vessels) decreases it.
Water always moves from an area of higher water potential to an area of lower water potential until equilibrium is reached.
Examples of Osmosis in Action
Osmosis plays a crucial role in various biological processes:
- Plant cell turgidity: Water moves into plant cells by osmosis, creating turgor pressure that keeps the cells firm and upright. Wilting occurs when water loss reduces turgor pressure.
- Water absorption by roots: Osmosis drives the uptake of water from the soil into the roots of plants.
- Maintaining blood volume: Osmosis helps regulate blood volume and pressure by controlling the movement of water into and out of blood vessels.
- Kidney function: Osmosis is essential for the reabsorption of water in the kidneys, regulating the concentration of urine.
Dialysis: Separating Substances Based on Size and Solubility
Dialysis, unlike osmosis, is a process that separates molecules based on their size and solubility. It doesn't rely solely on water potential differences. Instead, it uses a semi-permeable membrane with pores of a specific size to allow the passage of smaller molecules while retaining larger ones. The driving force behind dialysis is a concentration gradient (or a pressure gradient in some cases, such as in hemodialysis).
Types of Dialysis
Two primary types of dialysis are commonly discussed:
- Hemodialysis: This artificial kidney process is used to remove waste products and excess fluid from the blood of patients with kidney failure. Blood is passed through a dialyzer containing a semi-permeable membrane, where waste products diffuse from the blood into a dialysis fluid (dialysate). A pressure gradient often assists this process.
- Peritoneal dialysis: In this procedure, a dialysis solution is introduced into the peritoneal cavity (the abdominal cavity). Waste products and excess fluid diffuse from the blood across the peritoneal membrane into the dialysis solution, which is then drained.
Key Differences Between Osmosis and Dialysis
The table below summarizes the key differences between osmosis and dialysis:
| Feature | Osmosis | Dialysis |
|---|---|---|
| Driving force | Water potential difference | Concentration gradient (or pressure gradient) |
| Primary movement | Water | Solutes and water |
| Membrane selectivity | Primarily permeable to water | Permeable to molecules below a certain size |
| Process type | Passive transport | Can be passive or active (e.g., hemodialysis) |
| Purpose | Water balance, cell turgor, etc. | Waste removal, separation of molecules |
| Specificity | Not specific to particular solutes | Can be selective based on pore size |
Further Exploring the Nuances: A Deeper Look at Membrane Transport
Both osmosis and dialysis are crucial examples of membrane transport, a vital process in living organisms and various industrial applications. To fully appreciate their significance, let's explore the broader context of membrane transport mechanisms:
Passive Transport: Osmosis and Diffusion
Osmosis, as previously mentioned, falls under the category of passive transport. This means it doesn't require energy input from the cell. Another key example of passive transport is simple diffusion, where molecules move from an area of high concentration to an area of low concentration across a membrane without the assistance of membrane proteins. Both osmosis and simple diffusion are driven by the second law of thermodynamics, aiming to increase entropy (disorder) within the system.
Active Transport: Moving Against the Gradient
In contrast to passive transport, active transport requires energy, typically in the form of ATP (adenosine triphosphate), to move molecules against their concentration gradient (from low concentration to high concentration). This process often involves membrane proteins, such as pumps, that bind to and transport specific molecules. Examples include the sodium-potassium pump, vital for maintaining cell membrane potential.
Facilitated Diffusion: Protein-Assisted Passive Transport
Facilitated diffusion is another type of passive transport where membrane proteins facilitate the movement of molecules across the membrane. While it doesn't require energy, it's faster than simple diffusion and highly selective, allowing only specific molecules to pass through. This is crucial for transporting larger molecules or those that are not readily soluble in the lipid bilayer of the cell membrane.
Membrane Permeability and Selectivity
The semi-permeable nature of biological membranes is fundamental to both osmosis and dialysis. The selectivity of the membrane determines which molecules can cross and at what rate. This selectivity is determined by factors such as the size of the membrane pores, the chemical properties of the membrane, and the presence of specific transport proteins.
Applications of Osmosis and Dialysis: Beyond Biological Systems
The principles of osmosis and dialysis extend beyond the realm of biology, finding applications in various fields:
- Water purification: Reverse osmosis (RO) uses pressure to force water across a semi-permeable membrane, removing impurities. This technique is used for producing clean drinking water and purifying wastewater.
- Food processing: Osmosis is utilized in food preservation and processing, for example, in the dehydration of fruits and vegetables. Dialysis can be used in separating proteins and other components in the food industry.
- Medicine: Besides hemodialysis and peritoneal dialysis, osmosis and dialysis are involved in other medical procedures and technologies, including drug delivery systems and wound healing treatments.
- Industrial applications: Dialysis finds applications in various industrial processes, such as separating chemicals, purifying liquids, and concentrating solutions.
Conclusion: A Vital Understanding of Membrane Transport
Osmosis and dialysis, while both involving the movement of substances across membranes, differ significantly in their underlying mechanisms and driving forces. Osmosis is a passive process driven by water potential differences, focusing primarily on water movement. Dialysis, in contrast, separates substances based on size and solubility, driven by concentration or pressure gradients and often involving more complex membrane systems. Understanding these differences is crucial for comprehending a wide range of biological processes and various technological applications. A firm grasp of these concepts lays a strong foundation for deeper exploration into the fascinating world of membrane transport and its impact across various scientific disciplines. Further research into specific examples and applications will solidify this foundational understanding and open doors to further inquiry.
Latest Posts
Latest Posts
-
Is Water A Good Leaving Group
Mar 17, 2025
-
480 Cm Equals How Many M
Mar 17, 2025
-
What Is 8 In Fraction Form
Mar 17, 2025
-
The Elbow Is Proximal To The Shoulder
Mar 17, 2025
-
How Many Radians In A Revolution
Mar 17, 2025
Related Post
Thank you for visiting our website which covers about What Is The Difference Between Osmosis And Dialysis . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
