What Is The Charge Of Nickel
listenit
Mar 24, 2025 · 6 min read
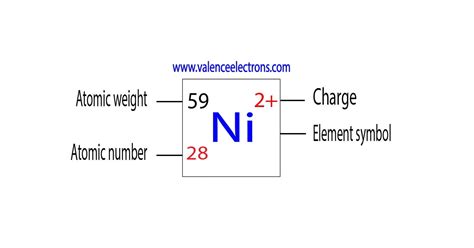
Table of Contents
What is the Charge of Nickel? Understanding Oxidation States and Reactivity
Nickel, a silvery-white metal with a lustrous appearance, is a fascinating element with a rich history and diverse applications. One key aspect of understanding nickel's behavior is its charge, or more accurately, its oxidation states. Unlike elements with a single, fixed charge, nickel exhibits multiple oxidation states, meaning it can exist with varying numbers of positive charges. This variability contributes significantly to its diverse chemical properties and its wide range of applications in various industries. This article will delve deep into the charges of nickel, exploring its common oxidation states, the factors influencing these states, and their implications in different chemical contexts.
Understanding Oxidation States
Before diving into nickel's specific oxidation states, let's clarify what oxidation state means. The oxidation state, also known as oxidation number, represents the hypothetical charge of an atom if all bonds to atoms of different elements were completely ionic. It's a crucial concept in chemistry, helping us predict the reactivity of elements and compounds. It indicates the number of electrons an atom has gained or lost in a chemical reaction. A positive oxidation state signifies the loss of electrons (oxidation), while a negative oxidation state indicates the gain of electrons (reduction).
Common Oxidation States of Nickel
Nickel's most common oxidation states are +2 and 0. However, it can also exhibit other oxidation states, although less frequently, including +1, +3, and +4. Let's explore each in detail:
Nickel(II) (+2): The Most Stable State
The +2 oxidation state is by far the most stable and common oxidation state for nickel. In this state, nickel has lost two electrons, leaving it with a +2 charge. This makes nickel(II) compounds abundant and readily available. Many nickel salts, like nickel(II) chloride (NiCl₂), nickel(II) sulfate (NiSO₄), and nickel(II) oxide (NiO), exist in this oxidation state. These compounds display a range of colors, depending on the ligands coordinated to the nickel ion. Nickel(II) complexes are often characterized by their paramagnetic nature, due to the presence of unpaired electrons.
Applications of Nickel(II): The prevalence of the +2 oxidation state makes nickel invaluable in various applications, including:
- Catalysis: Nickel(II) compounds are widely used as catalysts in various industrial processes, such as the hydrogenation of unsaturated fats and oils.
- Electroplating: Nickel plating is a common technique used to enhance the corrosion resistance and appearance of metallic surfaces.
- Batteries: Nickel-cadmium (NiCd) and nickel-metal hydride (NiMH) batteries rely on nickel(II) compounds for their functionality.
- Pigments and Ceramics: Nickel(II) compounds contribute to the vibrant colors of various pigments and ceramics.
Nickel(0): The Elemental State
Nickel in its elemental state (0 oxidation state) is a neutral atom with no net charge. This is the form in which nickel exists in its pure metallic state. Its applications stem from its inherent properties:
- Alloys: Nickel is a crucial component of numerous alloys, significantly enhancing their strength, corrosion resistance, and other desirable properties. Stainless steel, for instance, relies heavily on nickel's contributions.
- Coins and Currency: Nickel's durability and resistance to corrosion have led to its widespread use in coins and currency.
- Electronics: Nickel finds applications in electronic components, owing to its conductive and magnetic properties.
Less Common Oxidation States: +1, +3, +4
While less prevalent than +2 and 0, nickel can also exist in +1, +3, and +4 oxidation states. However, these are far less stable and often require specific conditions to form:
- Nickel(I) (+1): Nickel(I) compounds are relatively rare and are typically formed under highly reducing conditions. They are often characterized by their paramagnetism and strong reducing properties.
- Nickel(III) (+3): This oxidation state is less common than +2 but is observed in specific complexes with strong-field ligands. These ligands can stabilize the higher oxidation state. Nickel(III) compounds are often intensely colored and display significant oxidizing power.
- Nickel(IV) (+4): This is the least common oxidation state for nickel and is primarily observed in specific inorganic compounds, often those with highly electronegative ligands. These compounds are often highly oxidizing agents.
Factors Influencing Nickel's Oxidation State
Several factors influence the oxidation state that nickel adopts in a given chemical environment:
- Ligands: The nature of the ligands (atoms, ions, or molecules bonded to the central nickel ion) significantly influences the stability of different oxidation states. Strong-field ligands tend to favor higher oxidation states, while weak-field ligands favor lower oxidation states.
- pH: The pH of the solution can impact the stability of different nickel oxidation states. Acidic conditions can sometimes favor higher oxidation states.
- Reducing or Oxidizing Agents: The presence of strong reducing or oxidizing agents in the reaction environment can force nickel to adopt different oxidation states. Strong reducing agents favor lower oxidation states, while strong oxidizing agents favor higher ones.
- Temperature: Temperature can influence the stability and formation of different oxidation states. Higher temperatures might facilitate the formation of higher oxidation states under specific conditions.
Implications of Different Oxidation States
The various oxidation states of nickel have significant implications for its reactivity and applications:
- Reactivity: Different oxidation states impart different reactivities to nickel compounds. Higher oxidation states tend to be stronger oxidizing agents, while lower oxidation states can be stronger reducing agents.
- Catalysis: The ability of nickel to adopt different oxidation states makes it a versatile catalyst in many chemical reactions. The changes in oxidation state during catalysis allow it to facilitate electron transfer processes.
- Color: The oxidation state often dictates the color of nickel compounds. Nickel(II) compounds, for example, exhibit a wide range of colors depending on the ligand environment.
- Magnetic Properties: The number of unpaired electrons in nickel ions, determined by the oxidation state, influences the magnetic properties of the compounds.
Analytical Techniques for Determining Nickel Oxidation States
Several analytical techniques are employed to determine the oxidation state of nickel in a given sample:
- X-ray Photoelectron Spectroscopy (XPS): XPS can provide information about the core-level binding energies of nickel atoms, which can be correlated with the oxidation state.
- X-ray Absorption Spectroscopy (XAS): XAS is a powerful technique to probe the local electronic and geometric structure around nickel atoms, allowing determination of the oxidation state.
- Electron Paramagnetic Resonance (EPR) Spectroscopy: EPR is useful for characterizing paramagnetic nickel compounds, providing insights into the oxidation state and electronic structure.
- UV-Vis Spectroscopy: The characteristic absorption bands in the UV-Vis spectrum can sometimes provide information about the oxidation state of nickel complexes.
Conclusion
Nickel, a versatile transition metal, demonstrates a fascinating range of oxidation states, with +2 being the most common and stable. Understanding these oxidation states and the factors influencing their stability is crucial for predicting nickel's reactivity and its applications in various fields. The ability of nickel to readily transition between different oxidation states contributes to its significance as a catalyst and its use in diverse technological applications. Further research into nickel's oxidation states continues to expand our understanding of this important element and its potential in new and emerging technologies. The continuous exploration of its chemical properties ensures its ongoing relevance across many scientific and industrial sectors. From its role in essential alloys to its contribution in catalysis and energy storage, the multifaceted nature of nickel, driven largely by its variable oxidation states, continues to shape technological advancements worldwide.
Latest Posts
Latest Posts
-
Ground State Electron Configuration For Bromine
Mar 26, 2025
-
How Many Times Does 2 Go Into 19
Mar 26, 2025
-
What Is The Opposite Of Condensation
Mar 26, 2025
-
9 Oz Is Equal To How Many Cups
Mar 26, 2025
-
How Many Liters Is In 1500 Ml
Mar 26, 2025
Related Post
Thank you for visiting our website which covers about What Is The Charge Of Nickel . We hope the information provided has been useful to you. Feel free to contact us if you have any questions or need further assistance. See you next time and don't miss to bookmark.
